
Advanced technique of reduced-port proximal gastrectomy for gastric cancer
Introduction
Gastric cancer is one of the most common malignant diseases, and the second leading cause of cancer death worldwide (1). Since Kitano et al. first reported the successful induction of laparoscopy-assisted distal gastrectomy (LADG) for early gastric cancer, many clinical trials have unveiled the benefits of this technique (2-4). The conventional technique of laparoscopic gastrectomy usually requires five or six ports (5-7).
Reduced-port surgery (RPS), including single incision laparoscopic surgery (SILS) and needlescopic surgery (NS), in which fewer and smaller ports are used than in conventional procedures, is a novel surgical concepts that aim to decrease invasiveness and increased cosmetic benefits compared to conventional endoscopic surgery (8,9). Various RPS procedures have been reported in not only general surgery but also gynecology and urology (10,11). Several reports have described the involved surgical techniques and feasibility of RPS for gastric cancer. However, because of the technical difficulties coming from loss of triangulation and clashing of the forceps, RPS has scarcely been induced in laparoscopic gastrectomy, especially for upper third gastric cancer (12-14).
Proximal gastrectomy (PG) is a better choice of function-preserving surgery for upper third of early gastric cancer than total gastrectomy with respect to minimizing sequelae of gastrectomy and improving the quality of life after gastrectomy (15-18). Although various reconstruction methods after PG (e.g., esophagogastrostomy, double tract, jejunal pouch interposition, and jejunal interposition) have been reported, there is no consensus on the best reconstructive procedure. The most troublesome issues cropping up following PG with these reconstruction methods have been reflux symptoms induced by esophagogastric anastomosis and difficulty in endoscopic surveillance after surgery. To resolve these problems, Kamikawa et al. developed a unique esophago-gastrostomy procedure that incorporates a backflow valve (19). We used this method to perform RPS for PG to minimize the surgical invasiveness, improve cosmesis, and prevent postoperative reflux symptoms.
This chapter describes the techniques involved in our method of reduced-port proximal gastrectomy (RPPG) for gastric cancer using an oval-shaped port device and needlescopic forceps.
Indications and contraindications
Early gastric cancer located at upper-third stomach is a good indication for RPPG. For cT1cN0 tumors, the Japanese gastric cancer treatment guidelines recommends PG with D1+ lymphadenectomy for proximal cancer to conserve more than half of the distal stomach (20). We also included adenocarcinoma of the esophagogastric junction (AEG), tumors that were endoscopically classified as Siewert type II tumors, regardless of the depth of invasion.
Patients with morbid obesity (BMI <35), cancer invasion to the adjacent organs, and bulky tumor (<8 cm) should be excluded.
Technique
Positioning
The patient was placed in a reverse Trendelenburg position under general anesthesia. The surgeon stood by the patient’s right side. A scopist was positioned between the patient’s legs. An assistant surgeon positioned at the patient’s left side.
Port setting, access device, and needlescopic device
We adopted two patterns of trocar setting in our RPPG. One pattern involved a medium-sized single port device (21,22) (E-Z ACCESS oval type for LAP PROTECTORTM Oval type 0707D; Hakko Co. Ltd., Tokyo, Japan; Figure 1) with a scope trocar using 30-mm umbilical laparotomy and three 2.7-mm trocars (MiniportTM; Medtronic, Dublin, Ireland) in the right and left upper quadrants. The MiniportTM was introduced via percutaneous puncture. This port setting is known as NS (RPPG with three punctures), which means that the total number of trocars is the same as in conventional laparoscopic PG, but the size of the trocars is reduced (Figure 2).
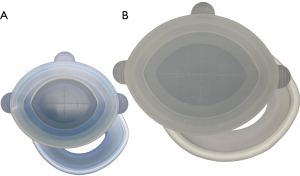
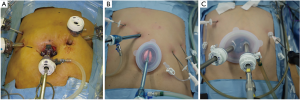
The other pattern involved a large-sized single-port device (E-Z ACCESS oval type for LAPPROTECTOR™ Oval type 1010HD, Hakko Co. Ltd.; Figures 1) with two 12-mm trocars in 30 mm umbilical laparotomy and two Miniports™ in the right and left upper quadrants (RPPG with two punctures, Figure 2C). In this setting, the number of assistant’s trocars was reduced from two to one. The surgeon’s right-hand trocar was changed from the right upper quadrant to the E-Z ACCESS oval type 1010HD to avoid a 12-mm skin incision in the right upper quadrant.
Needlescopic device (EndoRelief device, Hope Denshi Co. Ltd., Chiba, Japan), which has a 2.4 mm diameter shaft with a 5 mm diameter jaw, was adopted for our RPPG through Miniport™ (Figure 3A,B). Three EndoRelief devices were used in the former and two in the later port setting.
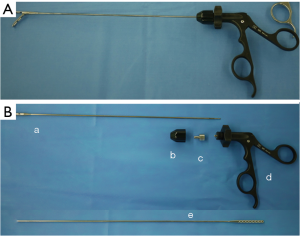
Of note: while the port settings are different, the operative procedure is the same for both port settings.
Liver retraction
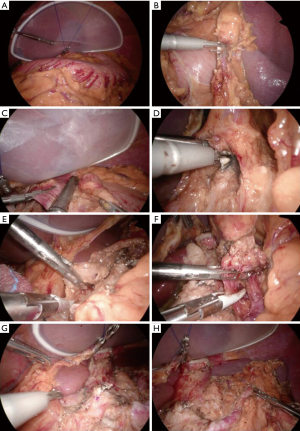
The liver retraction procedure without liver damage is essential to keep optimal surgical field of view during PG. A large sized Silicon-disc (Hakko Co., Tokyo, Japan) is inserted into the abdominal cavity and put under the lateral segment of the liver. The straight needle with the 2-0 thread is punctured through the skin at the left side of the epigastric region and introduced into an abdominal cavity. The needle is out through the skin at the right side of the epigastric region. A free jaw (FJ) clip (Figure 4, CHARMANT, Sabae, Japan) was introduced to the abdominal cavity. The thread was anchored to the gastrophrenic ligament with the FJ clip (Figure 5A). Extracorporeal bilateral ends of the thread were pulled and the liver was retracted with the thread and a silicon-disc. The ends of the thread were fixed with the clamps.

Procedure
RPPG with D1+ lymphadenectomy was performed according to the Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3) (20). A SonicBeat (Olympus Medical System Corp., Tokyo, Japan) and LigaSure Maryland (Medtronic., Dublin, Ireland) were used to facilitate lymph node dissection. Partial omentectomy began with the division of the greater omentum more than 3 cm from the gastroepiploic arcade to include LN station No. 4sb. The left gastroepiploic and short gastric vessels were sealed and divided to dissect LN stations No. 4sb and 4sa (Figure 5B). The greater omentum was divided to mobilize the distal stomach, but the right gastroepiploic vessels were preserved to maintain the blood supply to the distal stomach. The lower esophagus was divided intracorporeally with a laparoscopic linear stapler (Figure 5C). We performed the mobilization of the stomach and pancreas and the dissection of the cranial side of the station No. 9 lymph nodes prior to the medial approach (23). The suprapancreatic region (LN station No. 7) was then dissected. The coronary vein and left gastric artery were identified and divided after sealing or clipping (Figure 5D). The stations No. 8a and 11p lymph node along the common hepatic artery (Figure 5E) and proximal splenic artery (Figure 5F) were dissected. Following the completion of lymphadenectomy, the E-Z AccessTM oval type device was detached from the LAPPROTECTORTM to remove the gastric fundus (Figures 5G,H,6A). The stomach was transected under direct visualization. The resected proximal stomach was retrieved.
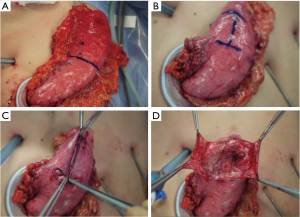
After PG, bowel continuity was restored by intracorporeal esophago-gastrostomy (hinged double flap method, Kamikawa’s procedure) (19,24). As this procedure was originally reported for laparotomic PG, we modified the procedure for laparoscopic surgery.
Pyoktanin blue marking (a sideways letter ‘‘H’’ sized 3.5 cm × 2.5 cm) was made on the remnant stomach (Figure 6B). Flaps were created by dissecting the submucosal and muscular layers approximately 2 cm from the top and right side of the anterior wall of the remnant stomach (Figure 6C,D). The remnant stomach was then returned to the abdominal cavity. Intracorporeal hand-sewn anastomosis was executed for reconstruction after recapping the device and reestablishing the pneumoperitoneum.
The top of the detached square of stomach tissue and the esophageal posterior wall (approximately 5 cm from the stump) were sutured with three intermittent 3-0 absorbable sutures (Figure 7A). A gastric incision for anastomosis was added 0.5 cm cranial from the bottom of the square region of the muco-submucosal layer (Figure 7B). The incision length was the same as the esophageal diameter. The esophageal stump was cut and opened for anastomosis.
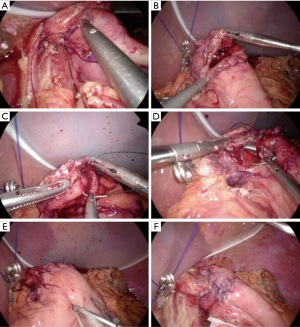
Regarding the esophago-gastrostomy of the posterior wall, the esophageal whole layer and the muco-submucosal layer of the stomach were sutured with running 3-0 absorbable barbed sutures (Figure 7C). After suturing the posterior wall, two-layered anastomosis was adopted for the anterior wall (Figure 7D,E). Continuous sutures using 3-0 absorbable barbed thread was used for the esophageal mucosa and gastric muco-submucosal layer suturing. Seven interrupted sutures were used for the esophageal muscle and gastric sero-muscular layer suturing (Figure 7E). The anastomosis was wrapped with the bilateral hinged flaps (Figure 7F). The bottom of each flap was sutured and attached with 3-0 absorbable barbed thread at the center. The bottom of the flap and the sero-muscular layer of the stomach were then sutured with running 3-0 absorbable barbed sutures. Each flap was then closed with four upward-running sutures. Finally, each flap was individually sutured to the anterior wall of the esophagus. The reconstruction was completed. Y-shaped flap wrapping was achieved at the end of the anastomosis. The pressure difference between the esophageal lumen and the gastric lumen creates a shutter mechanism at the anastomosis, which prevents esophageal reflux after surgery.
A 19 or 15 Fr silicone drain was inserted into the left subphrenic space after removing the trocar at the right upper quadrant, and was connected to a suction reservoir (Figure 8A).
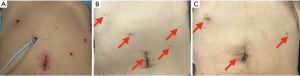
Postoperative abdominal wound and functional evaluation
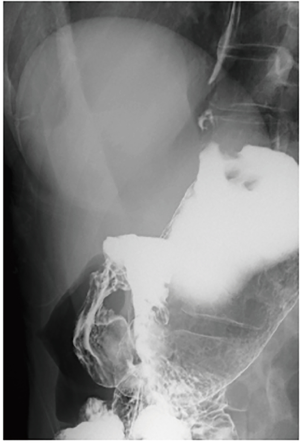
The umbilical wound was almost inconspicuous, and only pigmentation observed at the site of puncture by the Miniport™ one month after the surgery and will be invisible three month later (Figure 8B,C). Figure 9 showed upper GI series of the RPPG patient two months after the surgery. No esophageal barium reflux was observed even in the 15 degrees head-down tilt position.
Tips and tricks and recommendations from the author
NS, recognized as an important part of RPS, and has been developed as one of the surgical operations to replace conventional laparoscopic surgery (25,26). The recognized advantages of the NS are negligible scars, a potentially reduced pain, risk of port site hernias, and a lower incidence of wound complications. Therefore, using needlescopic forceps through 2- or 3-mm ports as the additional port in RPPG is very helpful for lymphadenectomy and reconstruction. In particular, the EndoRelief device, which has a 2.4 mm diameter shaft with a 5 mm diameter jaw, is worthy to introduce for RPS. With this forceps, probability of organ damage such as intestinal damage, which is a common problem with small diameter forceps, is very low. This forceps can be used in the same way as a regular 5 mm forceps, since this device has 5 mm diameter jaw.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chikara Kunisaki) for the series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. Shibao has received patent royalty from Hakko Co. Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005;19:168-73. [Crossref] [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Noshiro H, Nagai E, Shimizu S, et al. Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc 2005;19:1592-6. [Crossref] [PubMed]
- Uyama I, Sakurai Y, Komori Y, et al. Laparoscopy-assisted uncut Roux-en-Y operation after distal gastrectomy for gastric cancer. Gastric Cancer 2005;8:253-7. [Crossref] [PubMed]
- Kanaya S, Kawamura Y, Kawada H, et al. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer 2011;14:365-71. [Crossref] [PubMed]
- Gill IS, Advincula AP, Aron M, et al. Consensus statement of the consortium for laparoendoscopic single-site surgery. Surg Endosc 2010;24:762-8. [Crossref] [PubMed]
- Markar SR, Karthikesalingam A, Thrumurthy S, et al. Single-incision laparoscopic surgery (SILS) vs. conventional multiport cholecystectomy: systematic review and meta-analysis. Surg Endosc 2012;26:1205-13. [Crossref] [PubMed]
- Pfluke JM, Parker M, Stauffer JA, et al. Laparoscopic surgery performed through a single incision: a systematic review of the current literature. J Am Coll Surg 2011;212:113-8. [Crossref] [PubMed]
- Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc 2009;23:1419-27. [Crossref] [PubMed]
- Kunisaki C, Makino H, Yamaguchi N, et al. Surgical advantages of reduced-port laparoscopic gastrectomy in gastric cancer. Surg Endosc 2016;30:5520-8. [Crossref] [PubMed]
- Inaki N. Reduced port laparoscopic gastrectomy: a review, techniques, and perspective. Asian J Endosc Surg 2015;8:1-10. [Crossref] [PubMed]
- Lee CM, Park DW, Jung DH, et al. Single-Port Laparoscopic Proximal Gastrectomy with Double Tract Reconstruction for Early Gastric Cancer: Report of a Case. J Gastric Cancer 2016;16:200-6. [Crossref] [PubMed]
- Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg Today 2016;46:517-27. [Crossref] [PubMed]
- Hiki N, Nunobe S, Kubota T, et al. Function-preserving gastrectomy for early gastric cancer. Ann Surg Oncol 2013;20:2683-92. [Crossref] [PubMed]
- Koeda K, Nishizuka S, Wakabayashi G. Minimally invasive surgery for gastric cancer: the future standard of care. World J Surg 2011;35:1469-77. [Crossref] [PubMed]
- Takayama T, Matsumoto S, Wakatsuki K, et al. Novel laparoscopic procedure for treating proximal early gastric cancer: laparoscopy-assisted pylorus-preserving nearly total gastrectomy. Surg Today 2014;44:2332-8. [Crossref] [PubMed]
- Kamikawa Y, Kobayashi T, Ueyama S, et al. A new antireflux procedure in esophagogastrostomy after proximal gastrectomy. Shokakigeka 2001;24:1053-60.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Shibao K, Higure A, Yamaguchi K. Access Device 2: Multi-Channel Port. In: Mori T, Dapri G. editors. Reduced Port Laparoscopic Surgery. New York: Springer, 2014:44-56.
- Shibao K, Sato N, Higure A, et al. A new oval multichannel port to facilitate reduced port distal gastrectomy. Minim Invasive Ther Allied Technol 2015;24:135-40. [Crossref] [PubMed]
- Omori T, Oyama T, Akamatsu H, et al. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 2011;25:2400-4. [Crossref] [PubMed]
- Muraoka A, Kobayashi M, Kokudo Y. Laparoscopy-Assisted Proximal Gastrectomy with the Hinged Double Flap Method. World J Surg 2016;40:2419-24. [Crossref] [PubMed]
- Gagner M, Garcia-Ruiz A. Technical aspects of minimally invasive abdominal surgery performed with needlescopic instruments. Surg Laparosc Endosc 1998;8:171-9. [Crossref] [PubMed]
- Tagaya N, Abe A, Kubota K. Needlescopic surgery for liver, gallbladder and spleen diseases. J Hepatobiliary Pancreat Sci 2011;18:516-24. [Crossref] [PubMed]
Cite this article as: Shibao K, Murayama R, Hirata K. Advanced technique of reduced-port proximal gastrectomy for gastric cancer. Ann Laparosc Endosc Surg 2017;2:69.


