
Application of intercostal transthoracic trocars to laparoscopic hepatectomy
Introduction
Open liver resection for hepatic lesions in Couinaud’s segment VII or VIII (1) usually requires large skin incision extended to patient’s right abdominal wall, such as Mercedes incision, inverted L-shaped incision, and Makuuchi’s J-shaped incision with or without thoracotomy (2). Thus, laparoscopic approach to these hepatic regions can offer the best advantage over open liver resection in minimizing invasion to the abdominal wall. However, laparoscopic hepatectomy for segment VII or VIII lesions is technically difficult because these segments located in the cranial/dorsal ends of the liver, apart from a camera port and abdominal trocars (3,4).
In order to facilitate laparoscopic hepatectomy for the lesions in segment VII and/or VIII, Gayet developed “lateral approach”, i.e., the use of intercostal transthoracic trocars (ITT), enabling direct and vertical approach to the subphrenic hepatic regions (5). Recently, ITT begins to be used widely during wedge resections for subcapsular small tumors (6-8) as well as difficult hepatectomies for deeply-located tumors in segment VII and/or VIII (9). The aim of this study was to demonstrate technical details of the use of ITT in laparoscopic hepatectomy, with short-term outcomes in our latest series.
Methods
This study was conducted with the approval of our institutional ethics committee (No. 2016-1065). Informed consent was obtained from all of the patients.
Indication of ITT
ITT has been indicated to anatomic resections of hepatic segment VII and/or VIII (5), resection of deeply-located tumors requiring control of the major hepatic veins (9), and wedge resection of subcapsular hepatic tumors (6-8).
Insertion of ITT
Patients are placed in the low lithotomy position, with the legs spread apart and bent at the knees (French position). Then, the upper body of the patients is set in the left lateral decubitus position with the right arm suspended (Figure 1A,B). Following abdominal insufflation, three or four abdominal trocars are placed and the liver and tumor status is evaluated with a laparoscope and intraoperative ultrasonography. Then, balloon-tipped trocar(s), usually one 12 mm (caudal side) and one 5 mm (cranial side), are deployed from the intercostal space through the diaphragm in this order. Prior to the insertion of the intercostal trocars, ultrasonography should be used from patient’s body surface to confirm respiratory movement of the lower edge of the right lung. This lung movement can also be observed from the abdominal cavity and the diaphragm is compressed with the laparoscopic forceps, in order to exclude the lung from the pleural space around the trocar placement sites (Figure 1C). When the intercostal trocars are indicated in patients with previous surgery on the right lung, adhesions in the pleural cavity should be evaluated by using a transparent-type trocar and laparoscope as a thoracoscope, prior to the trocar insertion (Figure 1D). In order to prevent migration of pneumoperitoneum gas into the thoracic cavity, the intercostal trocars are fixed to thoracic wall by inflating the balloon of the trocars (Figure 1E).
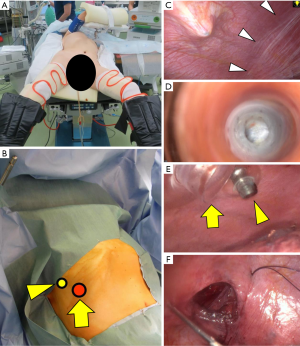
Hepatic mobilization
The operation table is moved to take reverse Trendelenburg and left semi-lateral position. The root of the right hepatic vein is confirmed following dissection of the falciform ligament and the right coronary ligament. Hepatic bare area is also dissected until the cranial and dorsal areas of hepatic transection line are fully exposed, but complete hepatic mobilization is unnecessary except for the case of anatomic segmentectomy or deep hepatectomy. Hepatic transection line is marked with electrocautery under ultrasound guidance. Fluorescence imaging using preoperative intravenous injection of indocyanine green (ICG) (10-12) is effective for identification of the subcapsular hepatic tumors during this process.
Hepatic transection
Operating surgeon stands on the patient’s right, using the cranial intercostal trocar by his/her left hand and the caudal intercostal trocar as a camera port. This position facilitates vertical transection of hepatic parenchyma around the tumor. In our center, hepatic parenchyma is dissected with cavitron ultrasonic surgical aspirator and vessel sealing systems under intermittent inflow occlusion. The intra-abdominal pressure is maintained at 12 mmHg throughout the procedures. When pneumoperitoneum gas continuously leaks into the thoracic cavity through the trocar placement site, a sheath of a 16 gauge intravenous needle should be placed from outside of the patient’s chest wall for preventing tension pneumothorax.
Wound closure
Fibrin glue is sprayed on the hepatic raw surface. The incisions on the diaphragm should be suture closed for preventing postoperative hernia. Incision for the caudal intercostal trocar can usually be closed from outside of patient’s thoracic wall with a Crochet hook needle without a risk of lung injury (Figure 1F). During suture closure of the diaphragm, an intercostal trocar should be kept in the pleural space for drainage of pneumoperitoneum gas leaking into the pleural cavity. Although we don’t use a chest drain routinely (13), postoperative chest X-ray is mandatory to confirm absence of progress of pneumothorax.
Results
From February 2012 to September 2016, totally 121 laparoscopic hepatectomies had been indicated at the Cancer Institute Hospital. Among them, ITTs were used in 17 patients (14%) with hepatic tumors in segment VII (n=6) or VIII (n=11). Twelve patients with segment VII or VIII lesions underwent laparoscopic hepatectomy without a use of ITT because tumors were located in caudal aspects of segment VII or VIII.
Surgeries were indicated as the initial hepatectomy except for a patient who had undergone open hepatectomy of segment VI and laparoscopic hepatectomy of segment II prior to the laparoscopic hepatectomy with the use of ITT. One patient had undergone video assisted thoracic surgery for right lung metastasis of rectal cancer before the laparoscopic hepatectomy with intercostal trocars. Two intercostal trocars were used in 10 patients (Figure 2). In the remaining seven patients, just a single 5 mm intercostal trocar was placed and used for operator’s left hand (Figure 3). The median (range) time required for insertion of intercostal trocar(s) was 3 [1–5] min.
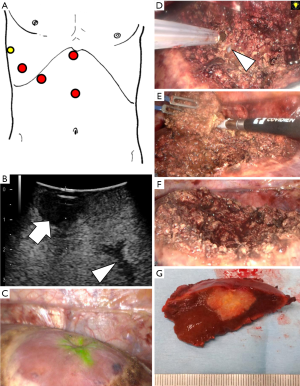
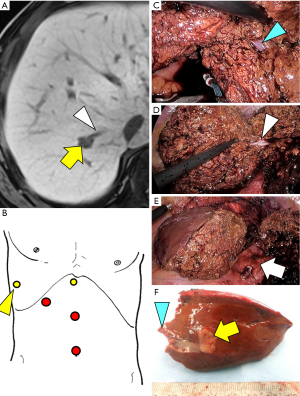
Median operation time and amount of blood loss for hepatectomy were 226 [109–565] minutes and 70 [3–680] mL, respectively. The maximum tumor diameter and the surgical margin were respectively 16 [6–25] and 10 [1–20] mm. Open conversion was required in one patient, who underwent open right paramedian sectoriectomy following laparoscopic resection of segment VIII because cancer invasion to the proximal Glissonian sheath was diagnosed by intraoperative rapid pathological examinations. No hepatectomy-related postoperative complications occurred, except for a case of intraabdominal abscess requiring operative drainage following simultaneous sigmoidectomy and hepatectomy for metastasis in segment II. The median (range) postoperative hospital stay was 8 [6–42] days.
Discussion
In order to employ minimally-invasive surgery to subphrenic hepatic lesions, several authors proposed thoracoscopic approach with incision on the diaphragm (14-16). However, the thoracoscopic transdiaphragmatic hepatectomy has not been applied commonly because of the limitations of this approach in completing intraabdominal surgical procedures such as Pringle maneuver and additional resection of the other hepatic regions. In 2012, Ishizawa et al. first reported laparoscopic hepatectomy with the use of ITT, enabling direct approach to the root of the middle and right hepatic veins during segmentectomy VII and VIII (5). Later, ITT began to be used more widely not only in “difficult” laparoscopic hepatectomy for deeply-located tumors (9) but for wedge resection of subcapsular tumors (6-8), as demonstrated in the present series (Table 1).
Table 1
| Reference | Procedures | Operation time, (min) | Blood loss, (mL) | Conversion, n (%) | Complication, n (%) | Maximum tumor size, (mm) | Surgical margin, (mm) | Postoperative hospital stay, (days) |
|---|---|---|---|---|---|---|---|---|
| Ishizawa et al. [2012] | Segmentectomy VII or VIII (n=7) | ND | ND | 0 (0) | ND | ND | ND | ND |
| Lee et al. [2014] | Segmentectomy VII or VIII (n=5) | 197±68* | 161±138* | 0 (0) | 0 (0) | 2.2±1.1* | 5.8±1.9* | 7±3* |
| Ogiso et al. [2014] | Resection of segment VII and/or VIII (n=25) | 217.5 [90–390]† | 200 [20–2,900]† | 0 (0) | 4 (16.0) | 24.5 [8–49]† | 3 [0–13]† | 7 [4–22]† |
| Chiow et al. [2015] | Wedge resection of segment VII and/or VIII (n=8) | 105 [50–150]† | 220 [50–300]† | 0 (0) | 0 (0) | 20 [6–34]† | 3.5 [1–11]† | 2 [1–4]† |
| Current series | Wedge resection of segment VII (n=6) or VIII (n=11) | 226 [109–565]† | 70 [3–680]† | 0 (0) | 1 (6.0) | 16 [6–25]† | 10 [1–20]† | 8 [6–42]† |
*, mean ± SD; †, median [range]. ND, no data.
The use of ITT in laparoscopic hepatectomy implies several advantages. First of all, this approach enables surgeons to identify tumors just beneath a laparoscope and make hepatic transection planes vertical to hepatic surfaces, avoiding tumor exposure on the hepatic raw surfaces. Reproduction of the hemispherical wedge resection such as open hepatectomy would be beneficial to patients from the standpoint of preserving hepatic parenchyma as much as possible for possible postoperative recurrence of hepatic tumors (17-19). Second, minimizing the extent of hepatic mobilization by using ITT may reduce a risk of postoperative bleeding/ascites and avoid severe adhesions around the liver at the time of re-hepatectomy for recurrence.
The essential step in the placement of ITT is to prevent lung injury. Usually, the first intercostal trocar is placed just above the right subcostal line. Thus, a risk of the lung injury during this procedure would be neglectable, as in the case of percutaneous biliary drainage. Even when the cranial intercostal trocar is placed, the right lung is usually located above the trocar placement site; however, ultrasonographic and laparoscopic examinations should be added to confirm respiratory movement of the lower edge of the right lung. During the placement of intercostal trocars, the pleural space should be compressed through the diaphragm with the laparoscopic forceps from the abdominal cavity for preventing migration of the right lung into the trocar sites. Although ITT can be applied even in patients with previous lung surgery by using a laparoscope for observation of the pleural cavity, severe pleural adhesions should be contraindication of this technique.
During hepatic transection of the cranial/dorsal regions, an operating surgeon stands on the patient’s right side, using the first intercostal trocar as a camera port and the second one for surgeon’s left hand. An operator may stand between the patient’s legs using the intercostal trocar for his/her left hand, when caudal aspects of the liver are dissected or just one intercostal trocar is used. Use of a robotic scope holder may reduce burden of a scopist and extend surgical working space in this trocar setting (8).
The use of ITT involves a risk of pneumothorax caused by leakage of pneumoperitoneum gas from the abdominal cavity, although this can be minimized by using balloon-tipped trocars. When this situation is suspected by changes in vital signs and/or appearance of pneumoderma, a sheath of a thick intravenous needle should be inserted from outside of the chest wall into the pleural cavity for drainage of pneumoperitoneum gas. In order to prevent postoperative diaphragmatic hernia, incisions on the diaphragm should be suture closed prior to completion of laparoscopic procedures.
In conclusion, ITT is an easy and safe surgical technique, which facilitates laparoscopic wedge resection of subphrenic hepatic tumors as well as complicated hepatectomy of segment VII and/or VIII.
Acknowledgments
Funding: This work was supported by grants from the Mochida Memorial Foundation for Medical and Pharmaceutical Research (T Ishizawa) and the Ministry of Health, Labour, and Welfare of Japan (T Ishizawa).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giovanni Battista Levi Sandri) for the series “Laparoscopic Liver Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.21). The series “Laparoscopic Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted with the approval of our institutional ethics committee (No. 2016- 1065). Informed consent was obtained from all of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Couinaud C. Le Foie: Études Anatomiques et Chirurgicales. Paris: Masson et Cie, 1957.
- Lim C, Ishizawa T, Miyata A, et al. Surgical Indications and Procedures for Resection of Hepatic Malignancies Confined to Segment VII. Ann Surg 2016;263:529-37. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Ishizawa T, Gumbs AA, Kokudo N, et al. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 2012;256:959-64. [Crossref] [PubMed]
- Lee W, Han HS, Yoon YS, et al. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci 2014;21:E65-8. [Crossref] [PubMed]
- Chiow AK, Lewin J, Manoharan B, et al. Intercostal and transthoracic trocars enable easier laparoscopic resection of dome liver lesions. HPB (Oxford) 2015;17:299-303. [Crossref] [PubMed]
- Ichida H, Ishizawa T, Tanaka M, et al. Use of intercostal trocars for laparoscopic resection of subphrenic hepatic tumors. Surg Endosc 2017;31:1280-6. [Crossref] [PubMed]
- Ogiso S, Conrad C, Araki K, et al. Laparoscopic Transabdominal With Transdiaphragmatic Access Improves Resection of Difficult Posterosuperior Liver Lesions. Ann Surg 2015;262:358-65. [Crossref] [PubMed]
- Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504. [Crossref] [PubMed]
- van der Vorst JR, Schaafsma BE, Hutteman M, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013;119:3411-8. [Crossref] [PubMed]
- Kudo H, Ishizawa T, Tani K, et al. Visualization of subcapsular hepatic malignancy by indocyanine-green fluorescence imaging during laparoscopic hepatectomy. Surg Endosc 2014;28:2504-8. [Crossref] [PubMed]
- Ishizawa T, Zuker NB, Conrad C, et al. Using a 'no drain' policy in 342 laparoscopic hepatectomies: which factors predict failure? HPB (Oxford) 2014;16:494-9. [Crossref] [PubMed]
- Teramoto K, Kawamura T, Takamatsu S, et al. Laparoscopic and thoracoscopic partial hepatectomy for hepatocellular carcinoma. World J Surg 2003;27:1131-6. [Crossref] [PubMed]
- Cloyd JM, Visser BC. Video-assisted thoracoscopic transdiaphragmatic liver resection for hepatocellular carcinoma. Surg Endosc 2012;26:1772-6. [Crossref] [PubMed]
- Hallet J, Soler L, Diana M, et al. Trans-thoracic minimally invasive liver resection guided by augmented reality. J Am Coll Surg 2015;220:e55-60. [Crossref] [PubMed]
- Cipriani F, Shelat VG, Rawashdeh M, et al. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J Am Coll Surg 2015;221:265-72. [Crossref] [PubMed]
- Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 2008;247:109-17. [Crossref] [PubMed]
- Mise Y, Aloia TA, Brudvik KW, et al. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann Surg 2016;263:146-52. [Crossref] [PubMed]
Cite this article as: Ishizawa T, Ichida H, Saiura A. Application of intercostal transthoracic trocars to laparoscopic hepatectomy. Ann Laparosc Endosc Surg 2017;2:48.


