Laparoscopic distal gastrectomy and Roux-en-Y reconstruction
Introduction
In recent years, laparoscopic distal gastrectomy has become common, and gastrectomy has gained consensus as a suitable treatment for early stage stomach cancer (1). The efficacy of laparoscopic gastrectomy for advanced cancer and total laparoscopic gastrectomy and proximal gastrectomy for upper gastric cancer is currently being studied (2). This paper will illustrate and provide a summary of laparoscopic gastrectomy with D1+ dissection performed for early stage gastric cancer that will be helpful when the procedure is introduced in various facilities. This paper will also present the Roux-en-Y reconstruction method.
Indications, setup and port settings
Laparoscopic distal gastrectomy with D1+ dissection is generally prescribed for clinical stage IA cancer that was diagnosed prior to surgery. However, the procedure can also be used for cancers that have progressed to a later stage, if dissection cannot be performed because of a patient’s clinical parameters.
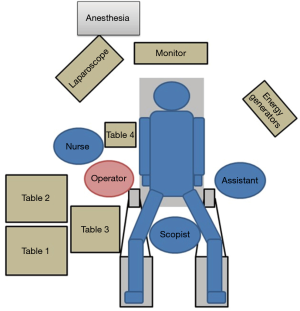
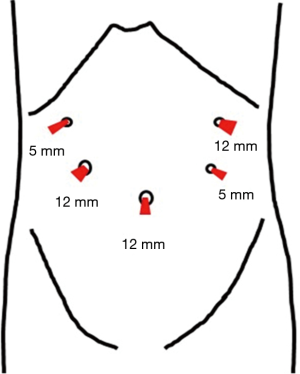
Before surgery, the patient’s body was placed in the supine position with the legs spread apart. It is generally best for the placement and setup of the surgical equipment if the hands are placed at the sides of the trunk. However, if this results in the i.v. line or sphygmomanometer being covered and managing the anesthesia may be problematic. Therefore, the decision as to where the hands should be placed needs to be made after discussion with the anesthesiologist. The monitor is commonly a single monitor, and it was placed at the head of the patient (Figure 1). There were five ports for the trocar: a 12-mm camera port at the navel, a 12-mm port below the left hypochondriac region, a 5-mm port on the left side of the abdomen, a 5-mm port on the right upper side of the abdomen, and a 12-mm port on the right side of the abdomen (Figure 2). All other devices were positioned as shown in the Figure 1.
Lifting the left hepatic lobe
Regardless of the device used, lifting up of the left lobe of the liver is required when working on the side of the lesser curvature of the stomach and during anastomosis. At the beginning of surgery, a Silicon DiscTM (Hakko Co. ltd., Nagano) was used and the left hepatic lobe was lifted with a straight needle and 2-0 nylon thread (Figure 3).

Distal gastrectomy and lymph node dissection D1+
This paper will describe the D1+ dissection technique for early gastric cancer.
Dissection of left greater omentum
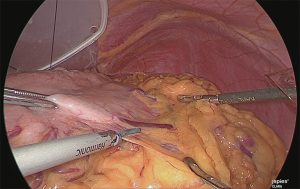
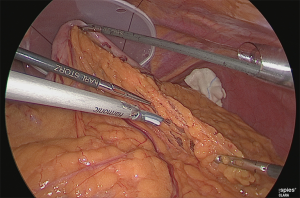
The assistant grasped the greater curvature of the stomach with the right hand and the greater omentum with the left hand. The greater omentum was separated while ensuring a 4 cm distance from the veins and arteries at the margin (Figure 4).
Handling of the veins and arteries of the greater omentum on the left side of the stomach (No. 4sb dissection)
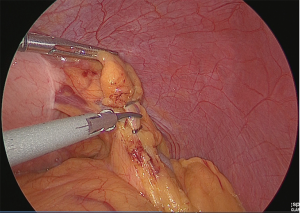
Clip separation was performed at the point where the gastroepiploic branch branches off from the veins and arteries of the greater omentum on the left side of the stomach (Figure 5).
Trimming
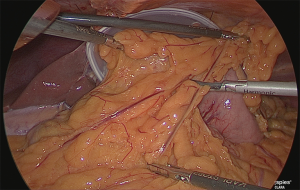
The greater omentum was trimmed up to the point where the gastric corpus would be resected (Figure 6).
Separation of the right side of the greater omentum
The surgeon changed position to stand on the left side of the patient. The assistant makes “matador-like” traction and the surgeon used his left hand to create a separated surface of the greater omentum and then proceeded to separate it progressively (Figure 7).
Pancreatic head lymph node dissection (No. 6 dissection)
After the colon was moved aside, the anterosuperior pancreaticoduodenal veins were exposed (foot-side boundary of the No. 6 lymph node) (Figure 8).
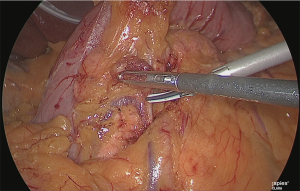
The assistant used his left hand to lift the veins and arteries of the greater omentum on the right side of the stomach, and his right hand to grasp and lift the posterior wall of the gastric antrum and he expanded the backside of the pyloric ring (Figure 9).
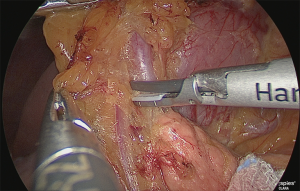
The layer outside the nerve (outer-most layer) of around the right gastroepiploic artery was secured, while expanding the detachable layer between the veins of the right gastroepiploic artery (Figure 10).
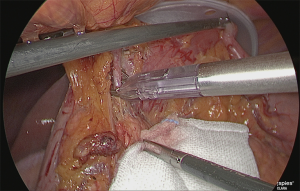
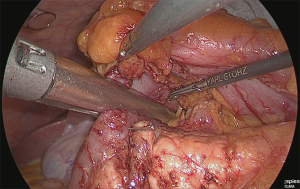
The right gastroepiploic artery was clipped and separated. Later, the adipose tissue, including the No. 6 lymph node that was at the time easily detachable, was dissected while retaining the detachable layer of the front surface of the pancreatic head, which had been ensured. Although the precise timing for incising the right gastroepiploic vein was not specified, performing the separation when the veins are under tension is believed to be appropriate (Figure 11).
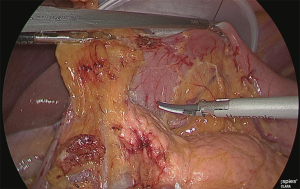
Separation of the duodenum
Gauze was inserted into the back side of the lesser curvature side of the duodenum; the gauze protruding from the abdomen side was released as a guide, and the veins and arteries of the upper duodenum were separated up to the point where the gastroduodenal artery could be confirmed (Figure 12).
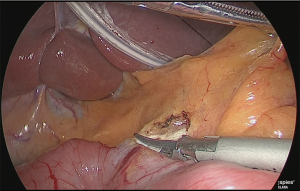
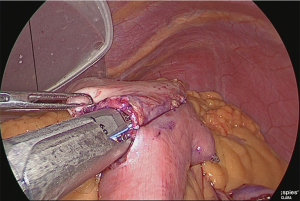
The duodenum was transected with a linear stapler (Figure 13).
Release of the lesser omentum
The surgeon returned to stand on the right side of the patient. The lesser omentum was released near the root of the right gastric artery and separated up to the lesser curvature of the stomach (Figure 14). At this time, the serous membrane was incised to the level of the first branch of the left gastric artery.
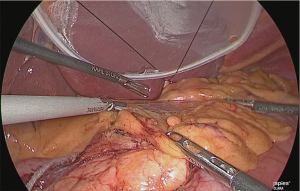
The serous membrane on the anterior surface of the crura of the diaphragm was incised with an electric scalpel (Figure 15), and the surface with which the myofascial layer would not be damaged was determined and this layer was expanded. This is the fusion fascia layer. Gauze for laparoscopic use was used to fill this layer to create a dorsal and temporal side receptacle for the dissected superior margin of the pancreas (Figure 16).
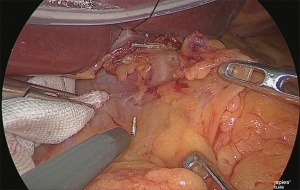
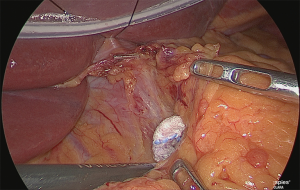
Handling of the right gastric artery
The assistant used his right hand to grasp and lift the pedicle of the right gastric artery and his left hand to grasp the surgical sponge, Securea™ (Hogy Medical, Tokyo, Japan), and he compressed the lower rim of the pancreas to turn the pancreas over (Figure 17).
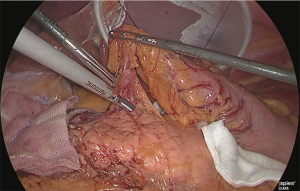
Using the outer-most layer of the common hepatic artery as the detachment surface, the root of the right gastric artery was peeled back while enlarging the surface; the root part was clip-separated (Figure 18). The assistant used his right hand to perform expansion to the left of the patient, and incised the serous membrane that lined the hepatic artery proper (in the case of a D2 dissection, that serous membrane is not cut and the layer outside of the nerve is directly detached to expose the left margin of the portal vein).
Dissection of the suprapancreatic lymph node
The assistant used his right hand to firmly grasp (activate the ratchet) and lift the gastropancreatic fold (pedicle of the left gastric artery), and with his left hand, turned the pancreas over by compressing the lower margin of the pancreas with a surgical sponge, Securea™ (Hogy Medical, Tokyo, Japan) (Figure 19).
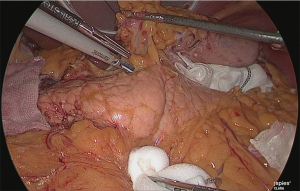
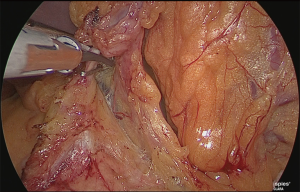
Detachment was performed in one continuous motion from the anterior surface of the common hepatic artery to the anterior surface of the splenic artery. One serous membrane on the left side of the gastropancreatic fold was incised at the level of the splenic artery. The so-called “ω-line” was ensured. The detachable layer outside the nerve on the left side of the left gastric artery was separated (Figure 20).
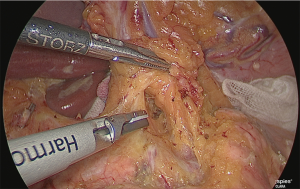
The gauze that was just used to fill the fusion fascia was reached. Both lateral sides of the left gastric artery were detached as much as possible to the back side. The crura of the diaphragm could be observed from this visual field, and if the detachable layer surrounding the celiac artery was exposed, the No. 9 lymph node was judged to have been lifted to the abdomen side.
The left side of the lymph nodes on the superior margin of the pancreas was cut out so that the internal and external detachable surfaces were sandwiched. To prevent damage to the pancreatic parenchyma and pancreatic artery, the positions where they were ascertained (Figure 21).
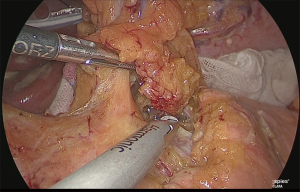
The periphery of the left gastric artery was clipped and separated (Figure 22).
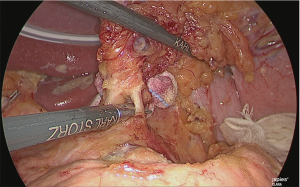
The base on the right side of the lymph nodes on the superior margin of the pancreas (near the boundary between No. 8a, 9 and 8p) was adequately sealed using the laparoscopic coagulating shears and separated (Figure 23).
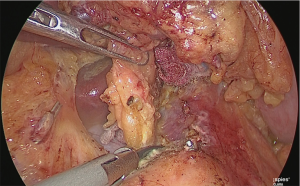
The dissection of the suprapancreatic lymph node was finished, and the posterior wall of the lesser curvature of the stomach was separated. At this point, if the vagus nerve were intact, it would have been cut out (Figure 24).
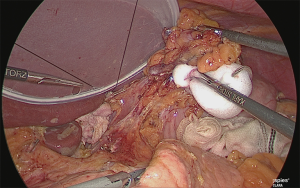
Trimming of the lesser curvature of the stomach (No. 1 and No. 3 lymph node dissection)
The stomach that was rolled up was returned, and the assistant lifted the lesser omentum with two hands in the manner of a matador and separated the lesser omentum from the gastric corpus from the back side of the lesser curvature of the stomach (Figure 25).
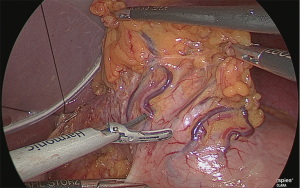
Next, the stomach was returned to its normal position, and the lesser omentum was separated from the gastric corpus on the abdominal side (Figure 26).
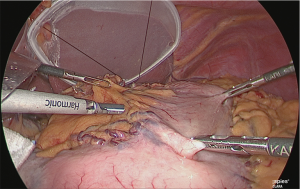
Separation of the gastric corpus
Using the linear stapler, the gastric corpus was separated. Normally, the gastric corpus is separated in two rounds (Figure 27).
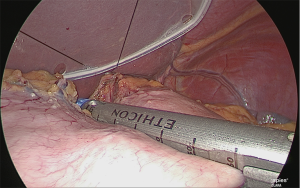
Extraction of the extracted stomach and re-insufflation
The extracted stomach was stored in a plastic bag (to prevent tumor dispersion and wound contamination) and extracted from the navel opening. During this time, the wound made was only large enough to perform the extraction and the enlargement was kept to a minimum. After extraction, a wound protector was inserted, a silicone cap was used as a cover, the camera port previously mentioned was inserted, and the stomach was re-insufflated (Figure 28).
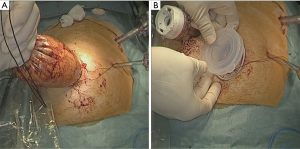
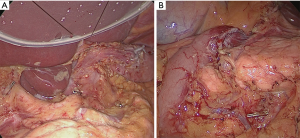
When all procedure of lymphadenectomy is finished, dissection area was checked (Figure 29).
Roux-en-Y reconstruction
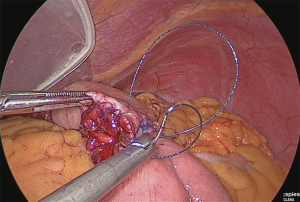
A small hole was made in the jejunal mesentery, at a part that was approximately 25 cm from the ligament of Treitz, and the jejunum was separated with the linear stapler. The mesentery was only slightly separated (separation of the arteries and veins at the margin) (Figure 30).
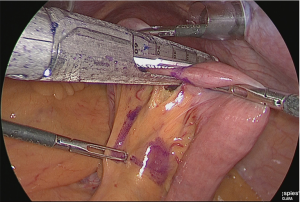
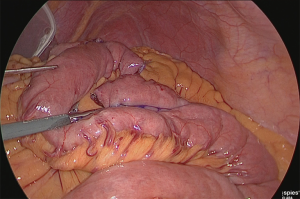
A small hole was made at the site approximately 25 cm from the tip of the lifted jejunum and at the tip of the afferent loop jejunum (Figure 31), and the linear stapler was used to perform side-to-side anastomosis (Figure 32). The insertion holes of the stapler were suture closed in a continuous single layer using 3-0 absorbent thread. The needle was handled from the far end of the visual field toward the near end of the visual field. As a trick for handling the needle, try to firmly stitch at the seromuscular layer and only slightly at the membrane surface (Figure 33).
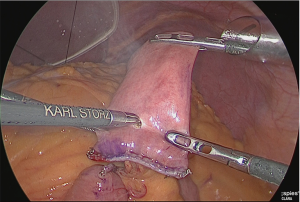
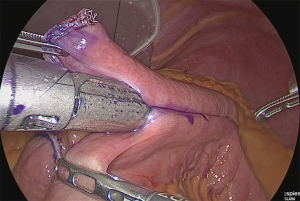

A small hole was made at the jejunum of Roux-limb (Figure 34).
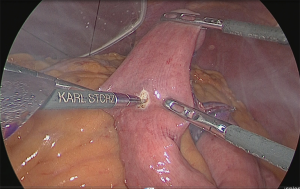
Anastomosis of the residual stomach and jejunum: the residual stomach and jejunum were anastomosed in an isoperistaltic manner. A small hole was made in the anal side approximately 60 mm from the tip of the lifted jejunum and in the tip of the lesser curvature side of the staple line of the residual stomach and anastomosis was performed using the linear stapler (Figure 35). The insertion holes of the stapler were suture closed using almost the same procedure as for the Y-limb (Figure 36).
Closure of Petersen’s space: a 3-0 non-absorbent thread was used to continuously suture close Petersen’s space. When doing this, the needle was handled from the front of the visual field to the back (from the leg side to the head side), and the space was suture closed up to near the colon. In patients in whom the greater omentum was conserved, the greater omentum would be gathered into the hands to perform stitching and the bottom rim would then be cut.
Closure of the Y-limb mesentery: the gap of the mesentery of the Y limb was continuously suture closed using non-absorbent thread. During this, the needle was handled so that the staple line of the resection stump of the jejunum was immersed under the mesentery as much as possible. This can be expected to prevent adhesion to the surface where the staples were exposed.
After the completion of reconstruction, the rout of reconstruction was inspected (Figure 37).
Depending on the circumstances, a 19 Fr closure-type drain should be placed from the right-upper 5-mm port to the lower surface of the liver, but at our facility, drain placement is not performed for D1+ dissection gastrectomies.
Post-operative management
The patient may begin drinking water two days after the operation. From day 3, soup is allowed; whole porridge and soft foods can be added, each at 2-day intervals. The patient can be discharged eight days after the operation.
Tips, tricks and pitfalls
When performing a dissection, close attention should be paid to keeping the visual field expansion and operative field dry. In addition to standardizing expansion, the electronic devices normally used to perform surgery are laparoscopic coagulating shears, Bipolar Maryland dissectors, and suction and water delivery devices with a button electrode.
With regard to reconstruction, the Y limb can usually be created from the small laparotomy surface in the navel. Depending on the capabilities of the surgeon, the abilities of the team, and the level of familiarity, when it is possible to do from the small laparotomy, it is recommended to be performed under direct visual guidance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chikara Kunisaki) for the series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.04). The series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Katai H, Sasako M, Fukuda H, et al. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 2010;13:238-44. [Crossref] [PubMed]
- Inaki N, Etoh T, Ohyama T, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg 2015;39:2734-41. [Crossref] [PubMed]
Cite this article as: Inaki N. Laparoscopic distal gastrectomy and Roux-en-Y reconstruction. Ann Laparosc Endosc Surg 2017;2:47.