
Anatomical laparoscopic right posterior sectionectomy
Introduction
Laparoscopic liver resection has evolved tremendously over the past two decades. It is shown to be associated with less wound pain, shorter hospital stay with a comparable oncological outcome with the open liver resection. Patients with solitary lesion, tumour less than 5 cm in diameter and located in the peripheral liver segments (i.e., Couinaud segments 2–6) are the best candidates for laparoscopic liver resection, as suggested in the First International Position on laparoscopic liver surgery published in 2008 (1). Due to the difficulty in bleeding control and visualization of the surgical field, lesions in the posterosuperior segments are generally considered not suitable for laparoscopic resection (2-4). With gaining experience and improvement in technology, the safety and feasibility of laparoscopic major resection, including those in the posterosuperior segments, have been published in recent years (4-9). Nonetheless, it is still considered in its experimental phase with incompletely defined risks in the Second Consensus Meeting held in Morioka (10). This manuscript aims to review the indications and the technical aspects of laparoscopic resection of the right posterior segments.
Anatomical resection
Anatomical resection is preferred for hepatocellular carcinoma (HCC), which has the propensity to invade the portal and hepatic veins, leading to intrahepatic metastasis. In fact, portal venous tumor extension and intrahepatic metastasis are two factors proven to be associated with poor prognosis (11-20). Anatomical resection is the systemic removal of the hepatic segment supplied by the tumor-bearing tributaries (21). Non-anatomical resection, on the other hand, may leave behind non-perfused ischaemic liver tissues and so it may not true parenchymal-sparing. Segment oriented anatomical resection preserves well-perfused non-tumour bearing liver parenchyma, which is important for patients with chronic liver diseases and cirrhosis. In the retrospective cohort study by Imamura, anatomical resection was shown to have significantly better recurrence-free survival (P=0.012) in a median follow-up of 480 days (22). Similar findings are also reported in other series (13,14).
On the other hand, there is no survival benefit for anatomical resection of colorectal liver metastasis. A meta-analysis of 5,207 patients showed that overall survival (hazard ratio 1.06, 95% confidence interval: 0.95–1.18) and disease-free survival (hazard ratio 1.11, 95% confidence interval: 0.99–1.24) did not differ significantly between anatomical resection and non-anatomical resection (23). The reason we still aim for anatomical resection even for selected cases of colorectal liver metastasis is that laparoscopic non-anatomical resection of segment 6 or 7 is a difficult procedure. Cho et al. reported that the operative time and blood loss for wedge resection of lesions in segment 6 or 7 were similar to those of major liver resection (24). This finding suggests that non-anatomical resection of the posterosuperior segment can be as difficult as major hepatectomy. It is also difficult to estimate and achieve an adequate resection margin for deep or large tumours located in the right posterior sections, despite frequent assessment by intraoperative ultrasound (17). Because of lack of tactile sensation through laparoscopy, by following the intersegmental plane along the right hepatic vein, resection margin can be better secured. Therefore, laparoscopic right posterior sectionectomy should be considered for deep and large lesions in the upper part of the right posterior sections of the liver. On the other hand, if the tumour is close to the right hepatic vein, conversion to right hepatectomy or extended right posterior sectionectomy with excision of the right hepatic vein should be contemplated to secure R0 resection.
Case selection
Careful patient selection is of paramount importance for the benefits of laparoscopic resection to be observed. In our center, we routinely perform indocyanine green retention (ICG) test and volumetry of the future liver remnant volume for all patients undergoing laparoscopic right posterior sectionectomy (8). We limit our indication of laparoscopic right posterior sectionectomy to tumor size up to 5 cm (1). For tumors that are not readily visualized on laparoscopy, anatomical resection and frequent use of intraoperative ultrasound (IOUS) to assess the resection margin is recommended. If the tumor is very close to the right hepatic vein, conversion to right hemihepatectomy or extended right posterior sectionectomy with excision of the right hepatic vein should be considered to secure complete resection. Cho et al. performed 24 laparoscopic right posterior sectionectomies with three open conversions (12.5%) due to inadequate tumor-free resection margin (9). This illustrates the importance of careful case selection in performing successful laparoscopic right posterior sectionectomy.
Operative technique
Glissonian approach for laparoscopic right posterior sectionectomy has the advantage of selective inflow control without jeopardizing the blood supply to the liver remnant. Parenchymal transection along the vascular demarcation of the right posterior sections and early identification of the right hepatic vein are the keys to precise anatomical resection. The techniques of retraction and exposure, meticulous dissection and secure hemostasis will be discussed.
Liver retraction and exposure
Gravity is a silent, obedient and reliable surgical assistant in liver retraction. Different patient positioning has been proposed in previous case series. In the case series by Tomishige et al. (25) and Cheng et al. (8), the left lateral position is adopted. The control of the Glissonian pedicle and liver parenchymal transection are performed without mobilization of the right lobe of liver. In this position, the vertical vector of the gravitational pull facilitates the visualization and approach of the transection plane from the caudal approach. Alternatively, the semi-prone position is used in the Ikeda series (26). The right triangular and coronary ligaments are divided and the weight of the liver helps to retract the liver from the diaphragm towards the left side. This creates space for the insertion of intercostal ports to facilitate parenchymal transection in the superior segments and control of hepatic vein branches. In addition, the Rouviere’s sulcus, the fissure housing the right posterior Glissonian pedicle, is readily seen after insertion of laparoscope in the semi-prone position. Nonetheless, these two positions place the operating surgeon in a non-ergonomic position on the left side of the patient. Therefore, the 30° semi-left lateral position with the lower limbs apart is proposed by the Korean group (9). In this way, the surgeon will be working in between the legs and the operating table can be tilted to the desired angle for the gravitational pull to work on liver retraction.
Parenchymal transection and bleeding control
Inflow and outflow control is important for correct anatomical resection and bleeding control. The intrahepatic Glissonian control is the mostly used method of inflow control (27). The right posterior Glissonian pedicle is approached via a hepatotomy along the fissure of Ganz with ultrasonic scalpel. The pedicle is then temporarily controlled with laparoscopic vascular clamp, which serves as a partial Pringle maneuver for bleeding control. An ischemic demarcation will then be seen and guide our parenchymal transection along the anatomical plane.
For outflow control, the right coronary and triangular ligaments need to be divided and the right lobe is mobilized from the inferior vena cava (IVC) until the root of the right hepatic vein (RHV) is found. Small hepatic veins branches are divided with clips or bipolar sealing device (9). The RHV is isolated and encircled with tape to allow prompt control of any brisk bleeding from the vein branches. Nevertheless, not all surgeons routinely perform outflow control and some prefer the anterior approach without mobilization of right lobe of liver for better retraction and visualization of the transection plane (8,25,28).
In addition, early identification of the RHV is important in guiding anatomical resection along the correct intersegmental plane. More bleeding is anticipated when we dissect close to the RHV. Having said that, parenchymal transection along the course of the RHV indicates the complete removal of the right posterior section without leaving devitalized liver tissue. Adhering to the right fissure is not easy as this is a curvilinear plane. The transection plane can be better visualized using flexible laparoscope and the course of the RHV can be clearly identified. Small hepatic vein branches are identified and controlled with clips or energy device. When bleeding is encountered, homeostasis can be readily achieved with bipolar diathermy, gauze compression or sutures (29). During parenchymal transection, the central venous pressure is kept low to less than 5 mmHg (30). When bleeding is encountered from the hepatic vein or even the IVC, the CO2 pneumoperitoneum can be increased to 15 to 20 mmHg to temporarily slowdown the rate of bleeding before applying energy device or suture for definitive control (31). Though CO2 gas embolism is one of the concerns from raising the intraperitoneal pressure, a swine model has demonstrated that this event occurs without much significant effects on the hemodynamic (32). By varying the intraperitoneal pressure during parenchymal transection, haemostasis can be readily accomplished with less blood loss in laparoscopic hepatectomy (31).
Various parenchymal transection techniques have been described in the literature. Ultrasonic scalpel is the most commonly used device (33). Other energy devices include cavitron ultrasonic surgical aspirator (CUSA), bipolar vessel sealer, monopolar sealer with saline tip and argon beam coagulator. Mechanical methods include crush clamp and stapler. Some reports use radiofrequency or microwave for pre-coagulation. In fact, the method of liver parenchymal transection is more of the surgeons’ preference and the evidence for the best technique is lacking. Nonetheless, there are some basic principles to uphold regardless of the energy device used. To minimize blood loss and maintain a clear surgical field, meticulous dissection and isolation of the intrahepatic vessels should be performed. These vessels can then be sealed and transected using clips, ultrasonic scalpel or cautery-based vessel sealer. Use of staples shall be limited to the transection of vascular pedicles.
Intercostal ports
The approach to the superoposterior segments of the liver is difficult from the caudal approach in laparoscopic hepatectomy. The addition of intercostal trocars as instrument port or camera port has been described to access the superior segments cranially (34-36). To avoid injury to the lung during insertion of the intercostal trocars, the cranial side of the diaphragm is compressed with the forceps introduced through the abdominal trocar (29). The intercostal trocars are then fixed to the thoracic wall by inflating the balloon to prevent migration of the pneumoperitoneum into the thoracic cavity (35). Camera can then be placed for direct vision of right hepatic vein by this lateral approach. The root of the hepatic vein can then be dissected and any bleeding here can be readily sutured (34). Upon completion of the operation, the diaphragmatic incisions will be closed with laparoscopic sutures from the caudal view and any remaining gas in the thoracic cavity is aspirated. Chest drain is usually not required.
Indocyanine green-fluorescence imaging
Indocyanine green (ICG) is excreted in bile and the excitation of the protein-bound ICG by non-infrared light cause it to fluorescent (37). This unique property makes ICG a very useful tool in hepatectomy.
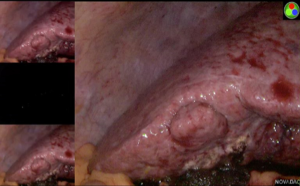
The Glissonian pedicle approach guides the parenchymal transection with an ischemic demarcation along the right fissure. However, this demarcation may be difficult to be visualized in patients with macronodular cirrhosis (Figure 1). To demonstrate the intended transection line along the anatomical plane, a counter-demarcation technique can be used (38). After temporary control of the right posterior pedicle with laparoscopic vascular clamp, 2.5 mg (0.5 mg per kg body weight) of ICG is injected intravenously. The right posterior section will be void of fluorescence and the demarcation can be clearly seen on the fluorescence laparoscopy (Figures 2 and 3).
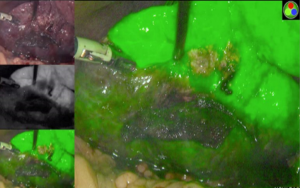
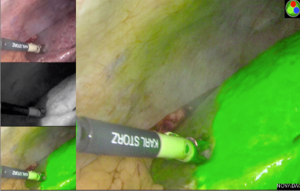
Secondly, ICG can be used for tumour staining due to its propensity to accumulate in HCC and in the non-cancerous liver parenchyma around metastatic adenocarcinoma (39,40). While ICG is readily taken up by differentiated HCC, the biliary excretion of ICG by the cancerous tissue is impaired, leading to retention of the fluorescence in the tumour. On the contrary, there is no uptake of ICG in liver metastasis and the biliary excretion of ICG by the surrounding non-cancerous hepatic parenchyma is also impaired, giving rise to a rim-type fluorescence. Such differential uptake of ICG and fluorescent pattern allow deep subcapsular lesions to be visualized on laparoscopy, enabling better margin control (38).
Furthermore, the biliary excretion of ICG can potentially help detecting bile leak over the resection surface. However, this novel technique requires further study before its widespread use in laparoscopic hepatectomy.
Hand-assisted laparoscopic hepatectomy and conversion
Similar to other laparoscopic surgeries, conversion should not be considered a failure. Conversion from pure laparoscopy to hand-assisted hepatectomy should be considered to control bleeding or to complete a difficult hepatectomy (1). Caution must be taken during conversion as the sudden loss of the pneumoperitoneum can result in massive hemorrhage. Therefore, attempts should be made to temporarily slowdown the bleeding by bipolar diathermy or compression with gauze packing before laparotomy. Surgeons embarking on laparoscopic resection should be facile with laparoscopic suturing and other techniques of hemorrhage control, negating the need to emergency conversion.
Operative outcome
Right posterior sectionectomy has been considered a relative contraindication to laparoscopic surgery because of perceived worse outcome when compared to resection of anterolateral segments (2,3). As experience accumulates, more recent series have shown comparable postoperative outcomes for different tumour locations. Cho et al. compared the outcomes of laparoscopic liver resection for 28 posterosuperior versus 54 anterolateral segments (4). They found that laparoscopic liver resection for tumors located in posterosuperior segments required longer operative time when compared with anterolateral segments (320 versus 210 minutes, P<0.001). There were no differences in the conversion rate, median blood loss, rate of intraoperative transfusion, median tumor-free margin, median hospital stay and complication rates between the two groups. Similar results were shown in another series by Kazaryan et al. published in 2011. In this series, no significant difference in the operative time was shown (41).
To our knowledge, there are only two case series on pure laparoscopic anatomical right posterior sectionectomy for HCC. Cheng et al. reported on the short-term outcomes of 13 patients undergoing laparoscopic anatomical right posterior sectionectomy for HCC (8). Up to one-third (30.8%) of these patients had cirrhosis on histology. The median operative time was 381 minutes with a conversion rate of 23%. The median resection margin was 8.7 mm and median hospital stay was 7 days. The conversion rate is comparable to previous reports for laparoscopic major hepatectomies (42-44), which have been shown to improve with experience (42,45). In the series by Cho et al., there was no difference in the mean resection margin and postoperative complications rate when compared with open surgery (9). However, the operative time was significantly longer in the laparoscopic group (567.4 vs. 316.1 minutes, P<0.001), but there was no significant difference in the length of hospital stay (10.6 versus 11.1 days, P=0.892). With the comparable short-term outcomes, laparoscopic right posterior sectionectomy is safe and feasible for experienced surgeons, yet technically demanding with longer operative time. It offers alternatives to right hepatectomy in selected patients if the functional liver remnant volume is inadequate (9).
Technical aspect aside, the long-term survival outcome of laparoscopic right posterior sectionectomy remains our primary concern. Nonetheless, it follows the oncological principle of anatomical resection and reports have shown comparable resection margin to open surgery. Whether these principles translate into clinical survival benefits is yet to be shown. Further prospective study with survival analysis will be needed in this regard.
Learning curve
One of the criticisms of laparoscopic hepatectomy is the lack of proper training and credentialing. One recent publication from France evaluated 173 patients for the learning curve for operating time using the cumulative sum (CUSUM) method (46). They stratified their experience into three phases: the initial learning curve consisting of 45 patients, followed by the plateau phase involving 30 patients with increased competency with laparoscopy, and lastly the mastery phase in which more complex procedures were performed with the subsequent 98 patients. Another study from England analyzed 159 patients who underwent laparoscopic hemihepatectomy (47). Risk-adjusted CUSUM analysis demonstrated a learning curve of 55 laparoscopic hemihepatectomies for conversions. However, these two studies did not include patient who underwent right posterior sectionectomies.
Cheng et al. studied on the learning curve for laparoscopic major hepatectomy analyzed 49 patients, including 13 laparoscopic right posterior hepatectomies (45). A shift in the average operative time was shown at the 25th case for laparoscopic major hepatectomy. In the subgroup analysis, the median blood loss for right posterior sectionectomy was significantly more than hemihepatectomy (1,500 vs. 500 mL, P=0.034); whereas the operative time, conversion rate, resection margin, complications and length of hospital stay were comparable. While the learning curve for laparoscopic right posterior sectionectomy is yet to be defined, this case series demonstrated the safety and feasibility of the procedure. Nonetheless, it is a technically demanding procedure and it should be performed by experienced hepatobiliary surgeons who have overcome the learning curve and proficient in laparoscopic hemihepatectomies.
Conclusions
Laparoscopic right posterior sectionectomy is technically demanding. Careful patient selection and preoperative planning cannot be emphasized more. The Glissonian approach as inflow control and transection along the correct intersegmental plane are the keys to anatomical resection. With the use of flexible laparoscope and various energy devices, a clear and bloodless surgical field can be maintained. Short term postoperative outcomes have been shown to be comparable with open operation. Further large scale prospective study is needed to define the long term oncological outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giovanni Battista Levi Sandri) for the series “Laparoscopic Liver Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.11). The series “Laparoscopic Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62. [Crossref] [PubMed]
- Laurent A, Cherqui D, Lesurtel M, et al. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 2003;138:763-9; discussion 769. [Crossref] [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 2008;144:32-8. [Crossref] [PubMed]
- Casaccia M, Andorno E, Di Domenico S, et al. Laparoscopic right posterior sectionectomy for hepatocellular carcinoma using a modified liver-hanging maneuver. J Laparoendosc Adv Surg Tech A 2012;22:488-91. [Crossref] [PubMed]
- Herman P, Krüger J, Lupinacci R, et al. Laparoscopic bisegmentectomy 6 and 7 using a Glissonian approach and a half-Pringle maneuver. Surg Endosc 2013;27:1840-1. [Crossref] [PubMed]
- Herman P, Krüger JA, Perini MV, et al. Laparoscopic hepatic posterior sectionectomy: a hand-assisted approach. Ann Surg Oncol 2013;20:1266. [Crossref] [PubMed]
- Cheng KC, Yeung YP, Ho KM, et al. Laparoscopic Right Posterior Sectionectomy for Malignant Lesions: An Anatomic Approach. J Laparoendosc Adv Surg Tech A 2015;25:646-50. [Crossref] [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Outcomes of laparoscopic right posterior sectionectomy in patients with hepatocellular carcinoma in the era of laparoscopic surgery. Surgery 2015;158:135-41. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Yamanaka N, Okamoto E, Toyosaka A, et al. Prognostic factors after hepatectomy for hepatocellular carcinomas. A univariate and multivariate analysis. Cancer 1990;65:1104-10. [Crossref] [PubMed]
- Franco D, Capussotti L, Smadja C, et al. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology 1990;98:733-8. [Crossref] [PubMed]
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992;69:913-9. [Crossref] [PubMed]
- Chen MF, Hwang TL, Jeng LB, et al. Hepatic resection in 120 patients with hepatocellular carcinoma. Arch Surg 1989;124:1025-8. [Crossref] [PubMed]
- Jwo SC, Chiu JH, Chau GY, et al. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection. Hepatology 1992;16:1367-71. [Crossref] [PubMed]
- Kosuge T, Makuuchi M, Takayama T, et al. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology 1993;40:328-32. [PubMed]
- Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg 1995;169:28-34; discussion 34-5. [Crossref] [PubMed]
- Shimada M, Takenaka K, Fujiwara Y, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein positive status as a new prognostic indicator after hepatic resection for hepatocellular carcinoma. Cancer 1996;78:2094-100. [Crossref] [PubMed]
- Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994;106:720-7. [Crossref] [PubMed]
- Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346-50. [PubMed]
- Imamura H, Matsuyama Y, Miyagawa Y, et al. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg 1999;86:1032-8. [Crossref] [PubMed]
- Tang H, Li B, Zhang H, et al. Comparison of Anatomical and Nonanatomical Hepatectomy for Colorectal Liver Metastasis: A Meta-Analysis of 5207 Patients. Sci Rep 2016;6:32304. [Crossref] [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Outcomes of laparoscopic liver resection for lesions located in the right side of the liver. Arch Surg 2009;144:25-9. [Crossref] [PubMed]
- Tomishige H, Morise Z, Kawabe N, et al. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J Gastrointest Surg 2013;5:173-7. [Crossref] [PubMed]
- Ikeda T, Toshima T, Harimoto N, et al. Laparoscopic liver resection in the semiprone position for tumors in the anteriosuperior and posterior segments, using a novel dual handling technique and bipolar irrigation system. Surg Endosc 2014;28:2484-92. [Crossref] [PubMed]
- Machado MA, Makdissi FF, Galvão FH, et al. Intrahepatic Glissonian approach for laparoscopic right segmental liver resections. Am J Surg 2008;196:e38-42. [Crossref] [PubMed]
- Liu CL, Fan ST, Cheung ST, et al. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg 2006;244:194-203. [Crossref] [PubMed]
- Ishizawa T, Gumbs AA, Kokudo N, et al. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 2012;256:959-64. [Crossref] [PubMed]
- Tranchart H, O'Rourke N, Van Dam R, et al. Bleeding control during laparoscopic liver resection: a review of literature. J Hepatobiliary Pancreat Sci 2015;22:371-8. [Crossref] [PubMed]
- Yeung YP, Cheng KC. Laparoscopic right hepatectomy with exposure of middle hepatic vein. Surgical Practice 2013;17:79. [Crossref]
- Jayaraman S, Khakhar A, Yang H, et al. The association between central venous pressure, pneumoperitoneum, and venous carbon dioxide embolism in laparoscopic hepatectomy. Surg Endosc 2009;23:2369-73. [Crossref] [PubMed]
- Otsuka Y, Kaneko H, Cleary SP, et al. What is the best technique in parenchymal transection in laparoscopic liver resection? Comprehensive review for the clinical question on the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2015;22:363-70. [Crossref] [PubMed]
- Ogiso S, Conrad C, Araki K, et al. Laparoscopic Transabdominal With Transdiaphragmatic Access Improves Resection of Difficult Posterosuperior Liver Lesions. Ann Surg 2015;262:358-65. [Crossref] [PubMed]
- Ichida H, Ishizawa T, Tanaka M, et al. Use of intercostal trocars for laparoscopic resection of subphrenic hepatic tumors. Surg Endosc 2017;31:1280-6. [PubMed]
- Schwarz L, Aloia TA, Eng C, et al. Transthoracic Port Placement Increases Safety of Total Laparoscopic Posterior Sectionectomy. Ann Surg Oncol 2016;23:2167. [Crossref] [PubMed]
- Scroggie DL, Jones C. Fluorescent imaging of the biliary tract during laparoscopic cholecystectomy. Ann Surg Innov Res 2014;8:5. [Crossref] [PubMed]
- Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr 2016;5:322-8. [Crossref] [PubMed]
- Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504. [Crossref] [PubMed]
- Ishizawa T, Masuda K, Urano Y, et al. Mechanistic background and clinical applications of indocyanine green fluorescence image of hepatocellular carcinoma. Ann Surg Oncol 2014;21:440-8. [Crossref] [PubMed]
- Kazaryan AM, Røsok BI, Marangos IP, et al. Comparative evaluation of laparoscopic liver resection for posterosuperior and anterolateral segments. Surg Endosc 2011;25:3881-9. [Crossref] [PubMed]
- Pearce NW, Di Fabio F, Teng MJ, et al. Laparoscopic right hepatectomy: a challenging, but feasible, safe and efficient procedure. Am J Surg 2011;202:e52-8. [Crossref] [PubMed]
- Dagher I, O'Rourke N, Geller DA, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg 2009;250:856-60. [Crossref] [PubMed]
- Vigano L, Laurent A, Tayar C, et al. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 2009;250:772-82. [Crossref] [PubMed]
- Chan FK, Cheng KC, Yeung YP, et al. Learning Curve for Laparoscopic Major Hepatectomy: Use of the Cumulative Sum Method. Surg Laparosc Endosc Percutan Tech 2016;26:e41-5. [Crossref] [PubMed]
- Nomi T, Fuks D, Kawaguchi Y, et al. Learning curve for laparoscopic major hepatectomy. Br J Surg 2015;102:796-804. [Crossref] [PubMed]
- van der Poel MJ, Besselink MG, Cipriani F, et al. Outcome and Learning Curve in 159 Consecutive Patients Undergoing Total Laparoscopic Hemihepatectomy. JAMA Surg 2016;151:923-8. [Crossref] [PubMed]
Cite this article as: Cheng KC, Ho KM. Anatomical laparoscopic right posterior sectionectomy. Ann Laparosc Endosc Surg 2017;2:36.


