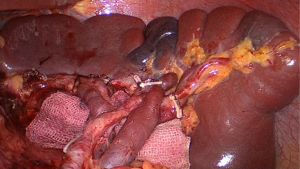
Strategies of laparoscopic spleen-preserving splenic hilar lymph node dissection for advanced proximal gastric cancer
Background
On the basis of the 14th edition of the Japanese gastric cancer (GC) treatment guidelines, a D2 lymphadenectomy is the normal surgery for advanced GC and No. 10 lymph nodes should be dissected for advanced proximal GC. Recently, the use of minimally invasive technology and the preservation of organ function are increasingly accepted by clinicians. Laparoscopic spleen-preserving splenic hilar lymph node dissection has become more identified in gastrectomy. As well as, it is critical that surgeons master flexibly the strategies of laparoscopic spleen-preserving splenic hilar lymph node dissection for a successful operation, as a consequence of the complexity of splenic hilar anatomy.
Value of spleen-preserving splenic hilar (No. 10) lymph node dissection for advanced proximal GC
Value of No. 10 LN dissection for advanced proximal GC
The foremost purpose of radical operations for GC is to prolong patients’ long-term survival. A patient’s postoperative survival rate is connected with the thoroughness of LN dissection during the surgery. No. 10 LN dissection is an important but difficult process of a D2 radical resection for advanced proximal GC. No. 10 LN metastasis rate has been reported to be 9.8–27.9% (1-3). Shin et al. (4) reported that of 319 patients with proximal gastric adenocarcinoma, 41 (12.9%) had No. 10 LN metastasis which is not apparent in early GC. Data from Japanese patients also revealed that the No. 10 LN metastasis rate in early proximal GC is so low (0.9%) that the No. 10 LN does not need to be dissected. However, in advanced GC, the No. 10 LN metastasis rate is 13.4% in stage T3 and 34.4% in stage T4 (5). A 346-case analysis of laparoscopic spleen-preserving No. 10 LN dissection for proximal GC conducted by our center showed that the incidence of No. 10 LN metastasis is 10.1%, and all of which had an advanced proximal GC (6). Age, tumor size, depth of invasion, No.4sb lymphatic metastasis are the independent risk factors of No. 10 LN metastasis. In addition, the rate of GC in the upper region is significantly higher than that in the lower third of the stomach (7). Koga et al. (8) reported that the incidence of No. 10 LN metastasis was highest in those patients with cancer of the whole stomach, Borrmann type IV or serosal invasion. Okajima et al. reported a highest No. 10 LN metastasis rate (26.7%) in GC of the entire stomach (9). Moreover, the survival rate is significantly associated with No. 10 LN metastasis. Shin et al. (4) reported that the 5-year survival rate was significantly lower for a No. 10 LN metastasis group (11.04%) compared with a non-metastasis group (51.57%) (P<0.05). Chikara et al. (10) reported that the 5-year survival rate of patients with No. 10 LN metastasis was 23.8%, whereas the rate in patients without No. 10 LN metastasis was 41.4% (P<0.05). Thus, the No. 10 LN metastasis status is a significant prognostic factor for GC. No. 10 LN dissection which is necessary in advanced upper GC because the radical excision of a tumor seems to be insufficient. Kosuga et al. (11) reported that there was no significant difference was found in the 5-year survival rates between patients with and without No. 10 LN metastasis (51.3% and 42.1%, respectively). Nonetheless, this study also suggested that patients with primary tumors localized on the greater curvature and Borrmann type 4 cancers might obtain a relatively high survival benefit from lymph node dissection at the splenic hilus by splenectomy. However, Ikeguchi et al. (12) reported a study of patients who underwent curative total gastrectomy with splenectomy in treatment of GC. The 5-year survival curves showed that the survival of No. 10 LN-positive patients did not differ from those of No. 10 LN-native patients. Therefore, the value of LN dissection in this area is significant. No. 10 LN dissection is becoming more accepted by an increasing number of clinicians.
Value of spleen-preserving No. 10 LN dissection for advanced proximal GC
Before the 1990s, the addition of splenectomy or pancreaticosplenectomy to total gastrectomy in patients with advanced GC aimed at facilitation of No. 10 LNs or No. 11 LNs dissection. Studies suggested that such dissection does not significantly improve the 5-year survival rate. There was not significantly different in the 5-year survival rate between the DP and DS groups (35.6% vs. 42.4%, P=0.6224). However, the morbidity rate was higher. Additionally, this surgical method increases the risk of distal pancreatectomy-associated complications such as pancreatitis, pancreatic fistula, intra-abdominal infection or abscess and postoperative diabetes. Currently, this surgical method is only performed when direct invasion of the spleen or of the body and tail of the pancreas is observed. Many subsequent studies have reported that the survival rates of patients undergone total gastrectomy in splenectomy group were higher than the spleen-preserving group (33.4% vs. 20.7%, P<0.05) (13). Moreover, compared with pancreatosplenectomy, pancreas-preserving splenectomy with No. 10 LN dissection has a similar survival rate and morbidity but significantly lower incidence of complications and mortality (14). Therefore, pancreas-preserving splenectomy with No. 10 LN dissection has been recommended as a curative procedure for standard D2 dissection instead of pancreatosplenectomy (9,15,16). However, Yang et al. (17) reported a meta-analysis of 466 patients showing that gastrectomy with splenectomy has not yet shown superiority on 5-year overall survival rate compare to splenic preservation, with an RR of 1.17 (95% CI: 0.97–1.41, P<0.05). Splenectomy could not facilitate prolongation of survival. This analysis also showed splenectomy had no significant influence on postoperative morbidity and mortality based on a 5-year overall survival rate outcome compared to splenic preservation for proximal and whole GC (RR =1.14, 1.76 and 1.58, respectively). Recently, many subsequent studies showed that the spleen is an important component of the peripheral immune system and it is the largest peripheral immune organ in the human body (18,19). The spleen contributes to normal operation of the circulatory system for immune regulation and plays roles in the immune and endocrine systems. Additionally, it contains numerous immune cells, the role of which in anti-tumor immunity is considerable. The Dutch scholar H.H. Hartgrink conducted a multicenter randomized controlled trial that followed up 1,078 patients with gastric adenocarcinoma for more than 10 years and found that splenectomy generated higher morbidity and mortality (20). In contrast, pancreas- and spleen-preserving LN dissection (D2) improved patient prognosis. Roderich et al. (21) reported that spleen-preserving No. 10 LN dissection is technically feasible based on the improvement of surgical techniques, with a curative effect similar to splenectomy. It lacks survival benefits after splenectomy (48.3% vs. 54.8%; P=0.503), but patient morbidity and mortality were significantly increased. Therefore, spleen-preserving No. 10 LN dissection is becoming more accepted by an increasing number of clinicians.
Value and procedure of laparoscopic spleen-preserving No. 10 LN dissection
Value of laparoscopic spleen-preserving No. 10 LN dissection
Along with the further study of the disease, the technique of surgery and surgical instruments has made tremendous progress. Hence, this operation tends to be more feasible and safer. Moreover, this is beneficial for reducing surgical trauma and protecting organ function. Traditional open operations can not no longer meet the needs of patients. Laparoscopic D2 LN dissections are conducted by many surgeons who have a good command of laparoscopic technology. Based on laparoscopic amplification and the superior effects of ultrasonic scalpels for cutting and hemostasis, surgeons can preferably clearly visualize the perigastric fascia, intrafascial space, vasculature, nerves and other structures, which makes the operation more smoothly. The splenic vessels and their branches can therefore be comfortably exposed, and the meticulous procedure of No. 10 lymphadenectomy can be carefully and efficiently completed. Therefore, spleen-preserving No. 10 LN dissection can get benefit from the use of laparoscopy. Hyung et al. (22) in 2008 firstly describe the detailed procedure of laparoscopic spleen-preserving splenic hilar LN dissection regarding the treatment of GC in the upper third of the stomach. A mean number of retrieved LNs at station 10 by laparoscopic spleen-preserving gastrectomy were similar to those by open gastrectomy (2.7 vs. 2.2). Besides, in laparoscopic operations, the surgery without mobilization of the spleen can achieve a smaller incision, minimal invasiveness and a shorter operating time. Furthermore, laparoscopic spleen-preserving No. 10 lymphadenectomy has been demonstrated technically feasible and safe. In our study, the average number of LNs retrieved using laparoscopic surgery was 3.6 per patient, and there was no patient required an open conversion because of an injury of the spleen or its vessels. No complications such as hemorrhage, splenic ischemia or splenic necrosis associated with dissection of the splenic hilar region were observed postoperatively, indicating favorable short-term outcomes (23). To date, however, the debate over the prognostic benefit of laparoscopic splenic hilar lymph node dissection for advanced GC is fierce. Hence, our center conducts a randomized controlled trial on laparoscopic spleen-preserving No. 10 lymph node dissection for advanced middle or upper third GC (No. NCT02333721). We believe that this trial could demonstrate the prognostic benefit of spleen-preserving No. 10 lymph node dissection. Moreover, we hope that this procedure has potential benefit for those patients without increasing in morbidity in experienced center.
Operative procedure of laparoscopic spleen-preserving No. 10 LN dissection
Some experts select a medial approach concerning the choice of surgical approach, the surgeon operates on the patient’s right side in which, and places an additional trocar below the xiphoid. Then, dissection of the No. 11p, 11d, and 10 LNs is performed with ultrasonic shears along the root of the splenic artery (SpA) toward its distal end. For a safe procedure, the surgeon cuts off the short gastric vessels (SGVs) prior to LN dissection, which is facilitated when the surgeon operates on the patient’s right side. Other experts choose a retropancreatic approach in which the surgeon operates on the patient’s left side and the assistant operates on the right. This procedure starts with separating the gastrosplenic ligament (GSL) and severing the left gastroepiploic and SGVs. The inferior border of the pancreas is then divided, which is better to entry into the retropancreatic space (RPS) and enable subsequent division of the SpA and splenic veins (SpV) and LN dissection within the RPS. For a better exposure, this approach requires removal of the entire stomach after severing the SGVs. However, this requirement violates the principle of an oncological en bloc resection. Moreover, as the appearance of metastatic No. 10 LNs, it may be inconvenient to expose anatomical plane and excise LN on account of inadequate traction on the GSL and the posterior wall of the gastric fundus. We enter the RPS through a left-sided approach, along the superior border of the pancreatic tail. We resect the LNs from the SLA toward the root of the SpA, and the SGVs are severed at their roots. This procedure, completing the removal of No. 10 LNs and the stomach, is consistent with the principle of oncological radical resection. Additionally, the assistant supports to providing better exposure by drawing the GSL, which maintains proper tension in the operative field.
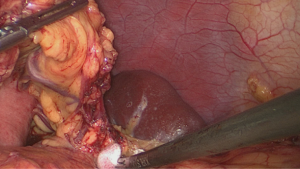
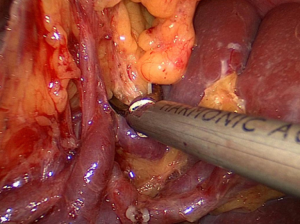
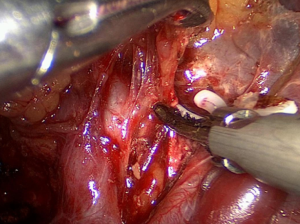
Our center completed more than 500 laparoscopic GC surgeries in January 2010, meanwhile, we performed laparoscopic spleen-preserving No. 10 LN dissections. Based on our experience, we have summarized an effective procedure called Huang’s three-step (24) maneuver for performing laparoscopic spleen-preserving No. 10 lymphadenectomies in clinical practice. The patient is placed in the reverse Trendelenburg position with his head elevated approximately 15 to 20 degrees and tilted left side up at approximately 20 to 30 degrees, which can better fully expose the superior border of the abdomen. The surgeon operates between the patient’s legs, the camera operator is on the patient’s right side just beside the left side of the operator, and the assistant is also on the patient’s right side. Step one is to dissect the LNs in the inferior pole of the spleen. The assistant puts the free omentum in the anterior gastric wall and uses his or her left hand to pull the GSL so that there is a greater space for operating .The surgeon gently presses the tail of the pancreas by gauze and separates the greater omentum along the superior border of the transverse mesocolon toward the splenic flexure of the colon (Figure 1).
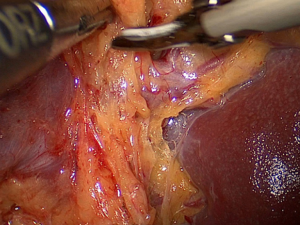
Next, along the direction of the pancreas, the surgeon peels the anterior pancreatic fascia (APF) off toward the superior border of the pancreatic tail (Figure 2).
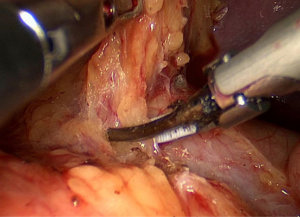
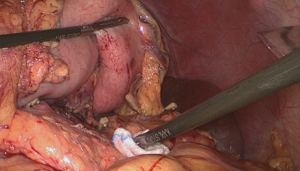
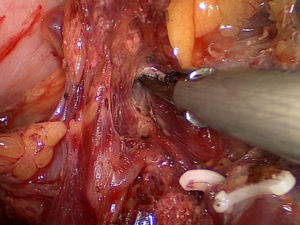
Then, the lower lobar vessels of the spleen (LLVSs) or lower pole vessels of the spleen can then be exposed which follows the peeled anterior lobe of the transverse mesocolon (ATM) and APF are completely lifted from the pancreas so that the RPS is entered (Figure 3).
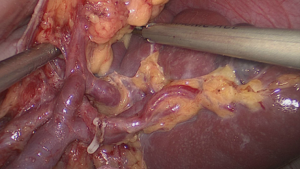
The assistant with his right hand lifts up the lymphatic fatty tissue on the surface of the vessels, and the surgeon dissects these lymphatic tissues by the non-functional face of the ultrasonic scalpel, sweeping toward the vessels. Therefore, the left gastroepiploic vessels (LGEVs) are revealed (Figure 4).
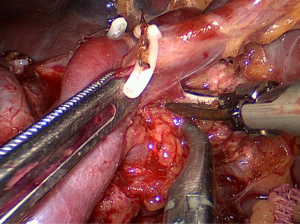
Then, the assistant pulls the LGEVs gently, and the surgeon separates the fatty lymphatic tissue meticulously from the LGEVs to completely denude them. The surgeon uses vascular clamps to divide the LGEVs at their roots (Figure 5).
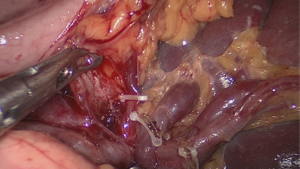
We transected 1 to 2 branches of the SGVs in the direction of the splenic hilum (Figure 6).
We should clamp the smallest amount of tissue possible, simultaneously, shear and divide the tissue step-by-step, which is preferable to reduce wound effusion. Avoiding excessive tension could prevent vessel tearing and uncontrollable hemorrhaging before the completion of coagulation and shearing with the ultrasonic scalpels.
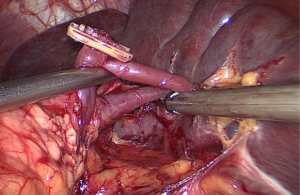
The second step is to dissect the LNs in the region of the SpA trunk. The assistant puts the free omentum between the inferior border of the liver and the anterior gastric wall and continually pulls the greater curvature of the fundus to the upper right, while the surgeon presses the body of the pancreas with his left hand (Figure 7).
The assistant with his right hand draws the isolated fatty lymphatic tissue on the surface of the SpA trunk. The surgeon denudes the middle of the SpA trunk along the latent anatomical spaces on the splenic vessel surface until the fork of the splenic lobar arteries lies along the latent anatomical spaces on the splenic vessel surface (Figure 8).
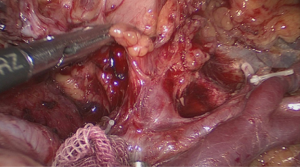
The posterior gastric artery (PGA) derived from the SpA, is always encountered in this region (Figure 9).
At this time, the assistant clamps and draws the vessels upward, and the surgeon denudes the vessels and closes toward the SpA trunk. Then, the surgeon divides the roots of the vessels with vascular clamps and dissects the fatty lymphatic tissue completely around the splenic vessels (No. 11d).
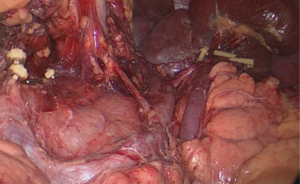
The third step is to dissect the LNs in the superior pole of the spleen. The assistant continually pulls the greater curvature of the fundus to the lower right, while the surgeon uses his left hand to gently press the vessels of the splenic hilum (Figure 10).
Then, the assistant, using the division point of the LGEVs as the starting point, gently pulls up the fatty lymphatic tissue at the surface of the terminal branches of the splenic vessels and keeps the tissue under tension while the surgeon cuts the surface of the terminal branches of the splenic vessels by the non-functional face of the ultrasonic scalpel.
This meticulous sharp or blunt dissection of the lobar vessels in the superior lobar area of the spleen completely skeletonizes the vessels in the splenic hilum (Figures 11,12).
In the time of the dissection procedure, two or three branches of the SGVs derives from the terminal branches of the splenic vessels and entering the GSL. Meanwhile, the assistant clamps and pulls the vessels upward, consequently, the surgeon particularly dissects the surrounding fatty lymphatic tissue, near the roots of the SGVs. After confirming the destinations of the roots in the stomach wall, the surgeon then divides the vessels at roots with vascular clamps. Especially, the last SGV in the superior pole of the spleen is usually too short that easy to damage, which results in bleeding (Figure 13). Subsequently, the assistant pulls the fundus to the lower right, which is better to completely expose the vessel and assist the surgeon with careful separation. Then, the separation makes it possible to complete dissection of the fatty lymphatic tissue in front of the splenic hilum.
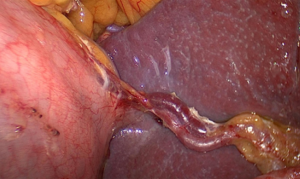
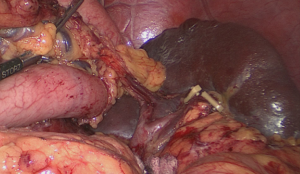
Dissection of the LNs behind the splenic vessels can be performed when the tail of the pancreas, which is a certain distance from the splenic hilum, is located in the inferior border of the spleen. The assistant, with his left hand, ventrally lift the termini of the splenic vessels by atraumatic grasping forceps, and the surgeon, with his left hand, press Gerota’s fascia (Figure 14). Then, the ultrasonic scalpel is applied to dissect the adipose tissue behind the splenic vessels and in front of Gerota’s fascia.
It is essential to be attentional during this step to prevent the separation plane from exceeding Gerota’s fascia. Exceeding Gerota’s fascia damages the kidneys and adrenal glands, as well as the related vessels or the nerves behind the vessels. By this time, the No. 10 lymphadenectomy is finished (Figures 15,16).
Strategies of laparoscopic spleen-preserving No. 10 LN dissection
The following points should be considered for safely and effectively performing laparoscopic spleen-preserving No. 10 LN dissection.
Importance of teamwork
Four instruments used by the surgeon and assistant are placed together in the location, deep and narrow, of the operative site within the abdominal cavity in the left upper abdomen. This phenomenon is known as the “chopsticks effect”. Hence, the camera assistant should adjust the orientation of the optical fiber and lens to avoid this problem. For our center, stable and automatic teamwork are known to be valuable in laparoscopic spleen-preserving No. 10 LN dissection. Above, we not only introduced the concrete operative steps taken by each participant but also indicated the scope and methodology of each technique to simplify the complicated No. 10 LN dissection procedure and to increase the efficiency of the operation. By presenting this information, we hope the procedure of laparoscopic spleen-preserving No. 10 LN dissection to be programmed, which will become prevalent in this field.
Step over the learning curve
The surgeon have to develop adequate and stable skills through an initial learning phase, called the learning curve of laparoscopic surgery, for performing laparoscopic spleen-preserving No. 10 LN dissection. This learning phase is usually measured by the number of surgical operations required before a beginner surgeon’s skills become relatively stable. Thus, when the surgeon achieves a certain number of surgeries, his or her operative technique will be significantly improved, typically reaching a plateau over time. Therefore, the surgeon has better mastered the technique. Indicators used to evaluate the learning curve include the operative time, blood loss, laparotomy rate, complication rate, time until first liquid diet, and length of postoperative hospitalization. With respect to achieving proficiency in laparoscopic GC surgical techniques, a surgeon can be regarded as a person with stable skills, following 40 surgical training procedures (25). To shorten the learning curve and ensure the safety, the surgeon should perform the procedure on patients in good condition who are younger with fewer complications, smaller tumors, and leaner Figures. These criteria reduce surgical risks, increase surgeon’s confidence and help them to eventually achieve proficiency. An adequate ability to summarize one’s experiences and lessons and to explore the operative position and anatomical approach that are most suitable for oneself will help the surgeon to gradually establish relatively stable surgical abilities.
Be familiar with the complex anatomy in this region
This area located in a narrow and very deep operating space adjacent to the splenic hilum is complex. The spleen usually adheres to the omentum or peritoneum, making it vulnerable to be injured, and even has a complicated relationship with the adjacent organs and tissues. Therefore, it is actually a bit difficult to expose the splenic hilar area, adequately and effectively. The dissection of No. 10 LN is in danger of injuring the splenic parenchyma or adjacent organs such as the pancreas and adrenal gland. In addition, the splenic vessels exhibit a tortuous course, and their branches are complicated, which may lead to a high risk of vessel injury and result in uncontrollable hemorrhage during laparoscopic dissection in this region. Therefore, we hypothesize that 3-dimensional computed tomography (3DCT) reconstruction can be used preoperatively to estimate the distribution of the splenic vessels. Indeed, this technique will reduce the difficulty and time-consuming nature of the surgery, as well as the risk of injury to the splenic hilar vessels, and even increase the surgeon’s confidence in the laparoscopic surgical technique. The research outcomes of our unit also revealed that compared with the non-3DCT group, the operative time and intraoperative blood loss were significantly decreased in the 3DCT group (26).
Prospect of laparoscopic spleen-preserving No. 10 LN dissection
Presently, in the evidence-based medical research although the long-term curative effect of laparoscopic spleen-preserving No. 10 LN dissection for advanced proximal GC is still not fully supported, the development of minimally invasive technology, represented here by laparoscopic technology, is an inevitable trend in GC surgery. Therefore, we should conduct a randomized controlled trial for spleen-preserving No. 10 LN dissection for advanced proximal GC to further confirm the efficacy of laparoscopic spleen-preserving No. 10 LN dissection.
Moreover, not all centers presently can independently complete laparoscopic spleen-preserving No. 10 LN dissection. Despite the substantial learning curve, surgeons at these centers must first master techniques for laparoscopic spleen-preserving No. 10 LN dissection. Grasp of these techniques is the key to completing the operation successfully. It is critical to provide professional training for GC surgeons, establish an active, hands-on training program and employ experienced and senior surgeons who can share their knowledge and experience to young doctors. Improving the level of integration of laparoscopic spleen-preserving No. 10 LN dissection is also a challenging target. Therefore, laparoscopic spleen-preserving No. 10 LN dissection will likely become one of the standard treatments for advanced proximal GC following improvement of the standardized operation training system and laparoscopic technology and the promotion of Huang’s three-step technique.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.11.14). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mönig SP, Collet PH, Baldus SE, et al. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol 2001;76:89-92. [Crossref] [PubMed]
- Sasada S, Ninomiya M, Nishizaki M, et al. Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res 2009;29:3347-51. [PubMed]
- Zhu GL, Sun Z, Wang ZN, et al. Splenic hilar lymph node metastasis independently predicts poor survival for patients with gastric cancers in the upper and/or the middle third of the stomach. J Surg Oncol 2012;105:786-92. [Crossref] [PubMed]
- Shin SH, Jung H, Choi SH, et al. Clinical significance of splenic hilar lymph node metastasis in proximal gastric cancer. Ann Surg Oncol 2009;16:1304-9. [Crossref] [PubMed]
- Sakaguchi T, Sawada H, Yamada Y, et al. Indication of splenectomy for gastric carcinoma involving the proximal part of the stomach. Hepatogastroenterology 2001;48:603-5. [PubMed]
- Huang CM, Zhang JR, Zheng CH, et al. A 346 case analysis for laparoscopic spleen-preserving no.10 lymph node dissection for proximal gastric cancer: a single center study. PLoS One 2014;9:e108480 [Crossref] [PubMed]
- Zhang CH, Wu AW, Li ZY, et al. Analysis of splenic hilar lymph node metastasis in advanced gastric cancer and dissection techniques. Zhonghua Wei Chang Wai Ke Za Zhi 2011;14:589-92. [PubMed]
- Koga S, Kaibara N, Kimura O, et al. Prognostic significance of combined splenectomy or pancreaticosplenectomy in total and proximal gastrectomy for gastric cancer. Am J Surg 1981;142:546-50. [Crossref] [PubMed]
- Okajima K, Isozaki H. Splenectomy for treatment of gastric cancer: Japanese experience. World J Surg 1995;19:537-40. [Crossref] [PubMed]
- Chikara K, Hiroshi S, Masato N, et al. Indications for pancreaticosplenectomy in advanced gastric cancer. Hepatogastroenterology 2001;48:908-12. [PubMed]
- Kosuga T, Ichikawa D, Okamoto K, et al. Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer 2011;14:172-7. [Crossref] [PubMed]
- Ikeguchi M, Kaibara N. Lymph node metastasis at the splenic hilum in proximal gastric cancer. Am Surg 2004;70:645-8. [PubMed]
- Yamamoto M, Baba H, Kakeji Y, et al. Postoperative morbidity/mortality and survival rates after total gastrectomy, with splenectomy/pancreaticosplenectomy for patients with advanced gastric cancer. Hepatogastroenterology 2004;51:298-302. [PubMed]
- Wang JY, Huang TJ, Chen FM, et al. A comparative study of pancreatectomy and pancreas-preserving gastrectomy in advanced gastric carcinomas. Hepatogastroenterology 2004;51:1229-32. [PubMed]
- Maruyama K, Sasako M, Kinoshita T, et al. Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 1995;19:532-6. [Crossref] [PubMed]
- Wang JB, Huang CM, Lu HS, et al. Efficacy of combined splenectomy in proximal gastric cancer with No.10 lymph node metastasis. Zhonghua Wei Chang Wai Ke Za Zhi 2009;12:121-5. [PubMed]
- Yang K, Chen XZ, Hu JK, et al. Effectiveness and safety of splenectomy for gastric carcinoma: a meta-analysis. World J Gastroenterol 2009;15:5352-9. [Crossref] [PubMed]
- Asteria CR, Gagliardi G, Pucciarelli S, et al. Anastomotic leaks after anterior resection for mid and low rectal cancer: survey of the Italian Society of Colorectal Surgery. Tech Coloproctol 2008;12:103-10. [Crossref] [PubMed]
- Penna Ch. Management of anastomotic fistula following excision of rectal cancer. J Chir (Paris) 2003;140:149-55. [PubMed]
- Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069-77. [Crossref] [PubMed]
- Schwarz RE. Spleen-preserving splenic hilar lymphadenectomy at the time of gastrectomy for cancer: technical feasibility and early results. J Surg Oncol 2002;79:73-6. [Crossref] [PubMed]
- Hyung WJ, Lim JS, Song J, et al. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 2008;207:e6-11. [Crossref] [PubMed]
- Li P, Huang CM, Zheng CH, et al. Laparoscopic spleen-preserving splenic hilar lymph node dissection for proximal gastric cancer. Zhonghua Wai Ke Za Zhi 2011;49:795-8. [PubMed]
- Huang CM, Chen QY, Lin JX, et al. Huang's three-step maneuver for laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer. Chin J Cancer Res 2014;26:208-10. [PubMed]
- Lu J, Huang CM, Zheng CH, et al. Learning curve of laparoscopy spleen-preserving splenic hilar lymph node dissection for advanced upper gastric cancer. Hepatogastroenterology 2013;60:296-300. [PubMed]
- Wang JB, Huang CM, Zheng CH, et al. Role of 3DCT in laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection. World J Gastroenterol 2014;20:4797-805. [Crossref] [PubMed]
Cite this article as: Zheng CH. Strategies of laparoscopic spleen-preserving splenic hilar lymph node dissection for advanced proximal gastric cancer. Ann Laparosc Endosc Surg 2017;2:31.