Sleeve and sleeve plus
Introduction
Obesity is an issue of epidemic concerns affecting all ethnicities, ages, and genders (1-3). In United States, about 35% of the adult population are considered to be obese (1,3). Obesity has strong relationship with numerous and various comorbid conditions such as diabetes, stroke, cardiometabolic diseases, obstructive sleep apnea (OSA), joint diseases (osteoarthritis), gallbladder problems and many cancers, as well as negative effect on quality of life (1-3). The rise of various bariatric surgeries have been well established in recent decades, especially for patients with morbid obesity, in terms of weight reduction, obesity-associated co-morbidities, and quality of life have been proved the effectiveness and safety (1,4-7). In addition, recent evidence has also shown bariatric surgery achieved better long-term survival than conventional medical treatments (8-12). The most common bariatric surgery procedures include laparoscopic Roux-en-Y gastric bypass (LRYGB), laparoscopic sleeve gastrectomy (LSG), laparoscopic adjustable gastric band (LAGB), and laparoscopic bilio-pancreasatic diversion with duodenal switch (LBPD-DS).
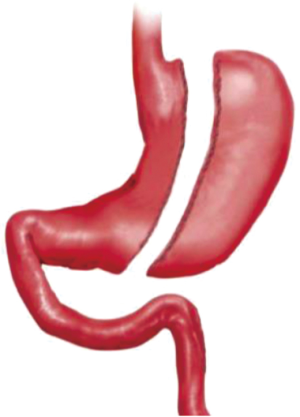
We acknowledge that the LRYGB (Figure 1) is the worldwide-accepted golden standard surgery for morbid obesity and type 2 diabetes mellitus (T2DM). However, its long-term complications such as marginal ulcer, dumping syndrome, iron deficiency anemia, Osteoporosis et al. make the birth of the LSG, which now is the primary and basic bariatric procedure. Sleeve gastrectomy is designed to be the restrictive component of BPD-DS in the beginning. For those high risk patients (ex. super-obesity or poor cardiopulmonary function patients), LSG was used as the first-step surgery prior to the two-stage procedure. It now becomes the primary one-step procedure (13-15). The simplicity of the procedure, effectiveness of weight loss, less postoperative morbidities and prominent resolution of comorbidities makes LSG become so popular. Although LSG has satisfied weight loss comparing with intensive medication therapy, LRYGB is still better than LSG on many espects such as the resolution of T2DM and results of weight reduction. In order to reach a comparable results with LRYGB without its disadvantages, we invented a new procedure: loop duodenojejunal bypass with sleeve gastrectomy (LDJB-SG).
LSG
LSG (Figure 2) provides desirable and quick weight loss with less vitamin deficiency (16). It involves only vertical resection of stomach of greater curvature side and creates a longitudinal and high pressured gastric tube.
Operative techniques
Mostly surgeons place 4–5 ports during performing LSG. Via the subxiphoid incision, a Nathanson’s liver retractor or our liver suspension technique are used to lift left hypertrophic liver (16,17). The gastroepiploic vessels are divided along the greater curvature. It begins 4–6 cm from the pylorus to the angle of His and left crus of diaphragm. And then along with a 38 French bougie, a vertical gastrectomy is performed with endoscopic staplers. The resected stomach is extracted via umbilical port. Single-incision trans-umbilical LSG also could be performed, which was associated with better cosmetic appearance, less need for analgesics, relatively scarcer complaints of postoperative pain, and more pleased over-all patients satisfaction compared with conventional multi-port LSG (17,18).
Weight loss results of LSG
Recent few years, more literatures are published to report weight loss results between intensive medication treatment, LSG and LRYGB. In the randomized controlled trial of Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) reported by Schauer et al. showed surgical groups had better percentage weight loss result from baseline, with weight reductions of 24.5%±9.1%, 21.1%±8.9% and 4.2%±8.3% in the gastric-bypass group, sleeve-gastrectomy group and medical-therapy group respectively (P<0.001 for both comparisons). And there was no statistically significance between LSG and LRYGB groups on % excess weight loss (%EWL) for 3 years follow-ups (19). Lee et al. found in veterans population, LRYGB achieved the most weight loss in kg, body mass index (BMI) reduction, %weight loss, and %EWL, followed by the LSG procedure, and AGB yielded the least weight loss (1). A 4-year weight change of multisite clinical veteran cohort study who underwent LRYGB, AGB and LSG showed weight loss compared with baseline BMI were 27.5%, 10.6%, 17.8% respectively (20) which revealed LRYGB had better weight loss. Other previous studies (21-24) also had the same conclusions of shorter-term weight loss (1–3 years).
For long-term weight regain, 30.5% for AGB; 14.6% for SG and 2.5% for LRYGB were observed (20,25). Arman et al. (26) recently showed a 11-year-follow-up outcomes of LSG, despite of the low follow-up rates (only 59%), the % excess body mass index loss was 62.5% in 11+ years. In our center, 63.71% EWL was reported at postoperative year 5 (27). Meta-analysis data also suggested that LRYGB resulted in a greater %EWL than LSG. It should be the size of the sleeve, the amount of antrum retained, and the amount of fundus resected that account for the variable weight loss results of LSG (28).
As for adolescent or young adult group, Maffazioli et al. found there was no statistically significant difference on body weight loss or weight regain after following up for 18 months in both LRYGB & LSG groups (29) which was consistent with Inge et al. (30) and Cozacov et al. (31) studies.
LSG has adequate and satisfied weight loss in both short-term and long-term results. Although LRYGB may showed superior in weight loss, its need of long-term vitamins supplement, nutritional and metabolic complications make patients backward.
Co-morbidities resolution after LSG
Morbid obesity attributes to many serious co-morbidities such as T2DM, dyslipidemia, sleep apnea, hypertension, osteoarthritis, blindness, amputation and gastro-esophageal reflux disease (GERD) (28). There are many therapies for morbid obesity including intensive medical therapy, behavior change, and even acupuncture, the alternative treatment. However, none of them can effectively resolve these problems or can provide the sustained success (19,28). Bariatric surgery results in not only the excellent outcome of sustained weight loss but also the advantage of comorbidities remission (32). More recently, many studies implicate bariatric surgery as a metabolic surgery because it also provides remission or improvement in T2DM in mildly obese patients (33-36).
Diabetes mellitus resolution result
In STEMPEDE trial, at 3 years, the target glycated hemoglobin level of 6.0% or less was achieved in 5%, 38%, 24% of the patients in the medical group, gastric-bypass group (P<0.001) and sleeve-gastrectomy group (P=0.01) respectively. There was also no statistically significant difference on LSG and LRYGB groups (19). According to Swedish Obese Subjects (SOS) study which comparing bariatric surgery with conventional medical treatment: the higher rates of diabetes remission at 2, 10 and 20 years and less long-term complications including total-cause mortality and major cardiovascular events were found in surgical treatment group (12,19,37,38). Some literatures showed LRYGB was still superior to LSG on resolution of insulin secretion and sensitivity. It’s also be the LRYGB, not the LSG, that reduce more truncal fat compared with subcutaneous fat (39). In our center experience, a 5-year-follow up of LSG showed 66.66% resolution of T2DM by definition of lesser or no use of diabetes medications (27). Nosso et al. found that for the morbid obese T2DM patients, in both LRYGB and LSG groups in terms of different hormonal and metabolic mechanisms involving in weight loss and T2DM remission one year after surgery, there were almost the same improvements of glucose profile in these two procedures. Weight loss is the key point of diabetes remission in morbidly obese T2DM patients one year after surgery (40).
It can’t be denied that weight loss changes the adipotoxicity in human body is the main cause of improvement of metabolic diseases in the early phase. However, there are more current data suggest that hormonal modulations, not weight loss alone, contribute to the beneficial effect of bariatric surgery for T2DM (41). LRYGB and LSG both change the islet function activity by altering enteroinsular axis. Gut hormones changes after LRYGB on ghrelin, peptide YY (PYY), and glucagon-like peptide-1 (GLP-1) are well documented (32). Similar to LRYGB, although to a lesser degree, LSG increases GLP-1 responses to meal ingestion (39,42) whereas gastric banding have no effect on postprandial glucose excursion or insulin and gut hormone responses (43). LSG indeed can improve T2DM to some extent.
Cardiovascular related markers resolution result
Hypertension
Among obese population, the most common co-morbidity is hypertension. Adipose tissue deposition can impair renal function and lead to blood pressure change (44). The possible mechanism is the altered neuroendocrine response, renin-angiotensin-aldosterone (RAA) system. LSG and LRYGB can result in a significant blood pressure reduction due to decreased cardiac stroke volume and lipotoxicity to the kidney after weight loss (45). In our center experience, a 5-year follow ups of LSG showed 100% resolution of hypertension (27). Otherwise, Li et al. meta-analysis showed the LRYGB is still more favored in remission rate of hypertension than LSG (28).
Dyslipidemia
From STEMPEDE trial, comparing medical and surgical treatments, 3-year-follow up showed much better sustained lower triglyceride and higher high-density lipoprotein (HDL) cholesterol levels in both LRYGB & LSG groups (19). In our 5-year-follow up of LSG, 50% resolution of hyperlipidemia was noted (27). Lee et al. reported 73.7% remission of dyslipidemia by a multicenter retrospective comparative cohort study of LSG (46). Li et al. meta-analysis showed 49.5% remission of hypertension of LSG compared with 71% of LRYGB (28).
OSA
Obesity is the most significant predisposing factor for OSA. According to de Sausa et al., elevation of 6 kg/m2 in BMI increases four times risks of developing OSA (47). The possible pathophysiological mechanisms of OSA are: (I) obese patients with OSA have 42% more fat in their neck, resulting in pharyngeal lumen narrowing; (II) leptin resistance which has a key role on controlling body-weight and respiratory center (48-50). A systemic review and meta-analysis made by Buchwald et al. reported OSA was resolved in 85.7% of obese patients with OSA (21). LRYGB is still the predominant choice of the surgery that has a better resolution rates than LSG (21,50,51). In fact, many literatures (50-53) discussed the differences in surgical efficacy maybe explained by weight-dependent and weight-independent effects (acronym BRAVE+ I: bile flow alteration, restriction of gastric size, anatomical gut rearrangement and altered flow of nutrients, vagal manipulation and enteric gut hormone modulation + improvement of systemic inflammation, such as soluble TNF-receptor 2, leptin which can increase neuromuscular control of pharyngeal diameter). Recent study done by Amin et al. revealed increase orexin levels after bariatric surgery is another possible weight-independent mechanism of early improvement of OSA (54). Both LRYGB and LSG can improve OSA in early phase of postoperative period (55). Dilektasli et al. reported sleeve gastrectomy can improve excess daytime sleepiness and sleep quality 6 months after the surgery (56). No matter what kinds of bariatric surgeries is chosen, OSA is a strong indication of bariatric surgery.
Nonacoholic fatty liver disease (NAFLD)
NAFLD is an important comorbidity of obesity and nonalcoholic steatohepatitis (NASH) is a precursor to the development of liver cirrhosis that may necessitate liver transplantation in the long run (57). Many literatures in recent years pay emphasis on the bariatric efficacy of improvement of NAFLD (29,58). There are no definite results on which type of surgeries has the best resolution rate. However, LSG seems to have a better improvement of liver function when comparing with LRYGB postoperative 6–12 months to date. According to Billeter et al., after 1 year follow up, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were both reduced in LRYGB and LSG groups. However, it’s the LSG group that has much lower AST and ALT levels than LRYGB and completely resolved the biochemical signs of NAFLD 12 months after the surgery (41% patients still had elevated ALT levels in LRYGB group) (58). Praveen et al. reported histological improvement of NAFLD within postoperative 6–8 months for both SG and LRYGB. However, SG appears to have a better effect on liver histology although this result did not reach statistical significance (59). In addition to weight loss, many experiments suggest change in bile acid metabolism and signaling through farsenoid-X receptor (FXR) which affects fatty acid metabolism of the liver may be the possible explanations of LSG on improvement of NAFLD (60-62).
Complications of LSG
There are early and late complications of LSG. When comparing with LRYGB, LSG is still a procedure with lower readmission and re-operation rates. Staple line leaks, bleeding, and strictures are the commonly reported complications following LSG. Shi et al. reported average rate of LSG complications in a systemic review: approximately 3.57% of bleeding rate, 12.1% of major complications, 1.17% of leak rate and mortality rates between 0 and 3.3% (63). International Sleeve Gastrectomy Expert Panel Consensus Statement 2011 [38] showed: 1.06% of leak rate, 0.35% of stricture with 1.05–1.85% of overall conversion rate and 3.66–5.1% of postoperative gastric fistula (64). LSG which as a longer staple lines has comparable leak rates to LRYGB which has shorter ones (65).
Gastroesophageal reflux disease (GERD) is the most common chronic complications complained by the patients and usually need to do revisional surgery which LRYGB is usually chosen. About 11–33% patients have GERD reflux in long-term follow ups (66) after LSG. Until now, there were no sufficient evidences to show the relationship between LSG and GERD. Chiu et al. (67) reported a systemic review showing no conclusive relationships on LSG to GERD. Keidar et al. implied when a relative narrowing of the middle stomach combined with a dilated upper stomach after the LSG, GERD may happen even without any complete obstruction (68). This functional obstruction would result in severe esophageal dysmotility with reflux symptom. Patients may reduce the incidence of GERD after LSG when they concomitant repair of hiatal hernia (HH) during the LSG operation (69). Preoperative evaluation of hiatal defects and repair of it during the LSG is recommended (70). For the small hiatal defect which is easily missed during preoperative panendoscopy examination, can be revealed and repaired easily when the surgeon remembers to exam the crura during LSG procedure and dissects left crura during dissection angle (66).
Why we need more in addition to LSG about diabetes resolution
LSG was once considered a restrictive procedure, but this presumption has recently come under scrutiny (71). It is found to be involved in “restriction”, “absorption” and “hormone change”. “Foregut” and “Hindgut” theories, recently even the midgut, can somehow give us possible explanations of LSG results. LSG resect the fundus of stomach where ghrelin is the main hormone to be secreted, which dramatically diminished and also increase the counter-hormone “obestatin” level in the early postoperative time (72). The counter-reaction of ghrelin and obestatin combined with decreased leptin may cause body to reduce appetite and utilize blood glucose effectively (73). Also postoperatively 1 year-follow up showed increase of CCK which also play a role in LSG on weight loss and sugar control (42). Up-regulated secretion of incretins (GLP-1, PYY), the glucose-dependent insulin enhancer, which were elevated while rapid delivery of partially digested food into distal intestine, combines with other changes mentioned above are important reasons to improve glucose tolerance after LSG (73-76).
Reduction of digestion was due to combination of restriction, the “appetite suppressive” effect from resection of the ghrelin-rich fundus, faster gastric emptying and decreased gastric acid secretion (77,78). Hormonal changes of LSG included antidiabetic effects of GLP-1 and PYY (79,80), which are not seen with the purely restrictive procedures like gastric banding. According to these studies, LSG can achieve satisfied body weight loss and T2DM resolution results. Otherwise, it is still inferior than LRYGB, which involves more physiologic mechanism of bypassing duodenum and proximal jejunum. To achieve better T2DM resolution, add foregut exclusion to sleeve gastrectomy (sleeve plus) might be an essential modification.
Sleeve plus: LDJB-SG
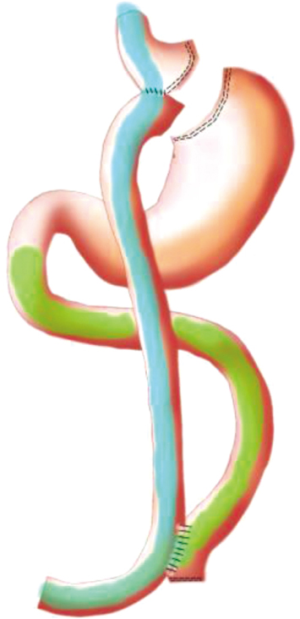
LRYGB and BPD-DS are procedures with higher rates of T2DM remission and long-term complications (81). The aim of metabolic surgery is to produce remission of T2DM with more physiological aspects and minimal morbidity and mortality. In our center, LDJB-SG (Figure 3), a novel surgical procedure was invented as a proposed technique for treatment of T2DM to reach the goal of metabolic surgery (82).
Operative techniques
Under general anesthesia, a 5-port laparoscopic surgery was used to access the abdominal cavity. We then performed a standard sleeve gastrectomy with endostaplers. After ensuring hemostasis, a stay suture was placed at the distal end of SG for counter-traction and better visualization of the first part of the duodenum. Two centimeters distal to the pylorus, we did the dissection of the duodenum. For firing the stapler, we need to use a tape to place for traction after the dissection of duodenum. We transected duodenum 2 cm from the pylorus, taking care not to injure the common bile duct (CBD), pancreas, and major vessels in the area. And then we measured 2–300 cm of the jejunal loop from the ligament of Treitz. We then performed side to side isoperistaltic, totally hand-sewn, one layered duodeno-jejunal anastomosis with absorbable sutures. After the anastomosis, we placed one anti-torsion suture in the antrum and upper jejunum, 4 cm proximal to the duodenojejunostomy. We then repaired the Peterson defect with a continuous non-absorbable suture. We put one Jackson-Pratt drain behind the duodenojejunal anastomosis reaching the sleeve and end the procedure (82).
Advantages of LDJB-SG
Exclusion of duodenum may ease the abnormal glycemic control and insulin resistant. Scientists found proximal bowel diversion, which was done on rat models, would not decrease food intake or weight loss but may improve diabetes instead. As previous elucidation, that’s the reason why LSG only resolved partial T2DM. Rubino et al. demonstrated when bypassing duodenum and proximal jejunum, amelioration of T2DM will occur without any change on food intake, body weight, malabsorption, or nutrient delivery to the hindgut (83).
LDJB-SG has higher satisfied T2DM resolution rates than LSG (remission rate for 1 year follow up: 62% vs. 32%) (84). For diabetes patients, surgery preserving the pylorus may cause delaying gastric emptying and then reduce postprandial glucose excursions (85,86). LDJB-SG is a good option for revision when intractable dumping syndromes happened after LRYGB (87). LDJB-SG also eliminates the risk of remnant gastric cancer, an important issue in Asia where gastric cancer is very common (88). Based on our experience, the resolution of co-morbidities was similar in both LDJB-SG and LRYGB for BMI <35 kg/m2 T2DM patients at postoperative 1 year. LDJB-SG has longer operative time and length of stay than LRYGB, however, it has no inferior rate than LRYGB on postoperative one-year improvement of body weight loss, fasting plasma glucose and %HbA1c. The level of HOMA-%B at 12 months was even significantly higher in the LDJB-SG than in the LRYGB (89). However, further studies on change of gut hormones and long-term results compared with RYGB, LDJBSG is still needed to be investigated in the future.
Conclusions
LSG has gradually taken place LRYGB as the main bariatric surgery in the world. And sleeve plus surgery, such as LDJB-SG, will become the main surgical procedure in treating obesity with T2DM, because of better resolution than LSG, but less complications than LRYGB.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.01.10). CKH serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jul 2016 to Jun 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee JH, Nguyen QN, Le QA. Comparative effectiveness of 3 bariatric surgery procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. Surg Obes Relat Dis 2016;12:997-1002. [Crossref] [PubMed]
- Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA 1999;282:1523-9. [Crossref] [PubMed]
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766-81. [Crossref] [PubMed]
- Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ 2014;349:g3961. [Crossref] [PubMed]
- Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg 2014;149:275-87. [Crossref] [PubMed]
- Karlsson J, Taft C, Ryden A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31:1248-61. [Crossref] [PubMed]
- Kolotkin RL, Davidson LE, Crosby RD, et al. Six-year changes in health-related quality of life in gastric bypass patients versus obese comparison groups. Surg Obes Relat Dis 2012;8:625-33. [Crossref] [PubMed]
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753-61. [Crossref] [PubMed]
- Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015;313:62-70. [Crossref] [PubMed]
- Maciejewski ML, Livingston EH, Smith VA, et al. Survival among high-risk patients after bariatric surgery. JAMA 2011;305:2419-26. [Crossref] [PubMed]
- Patterson EJ, Belle SH, Wolfe BM. Survival after bariatric surgery among high-risk patients. JAMA 2011;306:1323; author reply-4.
- Sjöström L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741-52. [Crossref] [PubMed]
- Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg 1998;8:267-82. [Crossref] [PubMed]
- Regan JP, Inabnet WB, Gagner M, et al. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg 2003;13:861-4. [Crossref] [PubMed]
- Sammour T, Hill AG, Singh P, et al. Laparoscopic sleeve gastrectomy as a single-stage bariatric procedure. Obes Surg 2010;20:271-5. [Crossref] [PubMed]
- Huang CK. Single-incision laparoscopic bariatric surgery. J Minim Access Surg 2011;7:99-103. [PubMed]
- Huang CK, Houng JY, Chiang CJ, et al. Single incision transumbilical laparoscopic Roux-en-Y gastric bypass: a first case report. Obesity surgery. Obes Surg 2009;19:1711-5. [Crossref] [PubMed]
- Saber AA, El-Ghazaly TH, Dewoolkar AV, et al. Single-incision laparoscopic sleeve gastrectomy versus conventional multiport laparoscopic sleeve gastrectomy: technical considerations and strategic modifications. Surg Obes Relat Dis 2010;6:658-64. [Crossref] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002-13. [Crossref] [PubMed]
- Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg 2016;151:1046-55. [Crossref] [PubMed]
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. [Crossref] [PubMed]
- Carlin AM, Zeni TM, English WJ, et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg 2013;257:791-7. [Crossref] [PubMed]
- Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011;254:410-20; discussion 20-2. [Crossref] [PubMed]
- Puzziferri N, Roshek TB 3rd, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA 2014;312:934-42. [Crossref] [PubMed]
- Ponce J, Nguyen NT, Hutter M, et al. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in the United States, 2011-2014. Surg Obes Relat Dis 2015;11:1199-200. [Crossref] [PubMed]
- Arman GA, Himpens J, Dhaenens J, et al. Long-term (11+years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 2016;12:1778-86. [Crossref] [PubMed]
- Zachariah SK, Chang PC, Ooi AS, et al. Laparoscopic sleeve gastrectomy for morbid obesity: 5 years experience from an Asian center of excellence. Obes Surg 2013;23:939-46. [Crossref] [PubMed]
- Li J, Lai D, Wu D. Laparoscopic Roux-en-Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy to Treat Morbid Obesity-Related Comorbidities: a Systematic Review and Meta-analysis. Obes Surg 2016;26:429-42. [Crossref] [PubMed]
- Maffazioli GD, Stanford FC, Campoverde Reyes KJ, et al. Comparing Outcomes of Two Types of Bariatric Surgery in an Adolescent Obese Population: Roux-en-Y Gastric Bypass vs. Sleeve Gastrectomy. Front Pediatr 2016;4:78. [Crossref] [PubMed]
- Inge TH, Courcoulas AP, Jenkins TM, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med 2016;374:113-23. [Crossref] [PubMed]
- Cozacov Y, Roy M, Moon S, et al. Mid-term results of laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass in adolescent patients. Obes Surg 2014;24:747-52. [Crossref] [PubMed]
- Sundbom M. Laparoscopic revolution in bariatric surgery. World J Gastroenterol 2014;20:15135-43. [Crossref] [PubMed]
- Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care 2012;35:1420-8. [Crossref] [PubMed]
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240-9. [Crossref] [PubMed]
- Liang Z, Wu Q, Chen B, et al. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013;101:50-6. [Crossref] [PubMed]
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567-76. [Crossref] [PubMed]
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93. [Crossref] [PubMed]
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56-65. [Crossref] [PubMed]
- Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 2013;36:2175-82. [Crossref] [PubMed]
- Nosso G, Griffo E, Cotugno M, et al. Comparative Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Glucose Homeostasis and Incretin Hormones in Obese Type 2 Diabetic Patients: A One-Year Prospective Study. Horm Metab Res 2016;48:312-7. [Crossref] [PubMed]
- Ochner CN, Gibson C, Shanik M, et al. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes (Lond) 2011;35:153-66. [Crossref] [PubMed]
- Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 2012;22:740-8. [Crossref] [PubMed]
- Korner J, Bessler M, Inabnet W, et al. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007;3:597-601. [Crossref] [PubMed]
- Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens 2010;23:1170-8. [Crossref] [PubMed]
- Sarkhosh K, Birch DW, Shi X, et al. The impact of sleeve gastrectomy on hypertension: a systematic review. Obes Surg 2012;22:832-7. [Crossref] [PubMed]
- Lee SK, Heo Y, Park JM, et al. Roux-en-Y Gastric Bypass vs. Sleeve Gastrectomy vs. Gastric Banding: The First Multicenter Retrospective Comparative Cohort Study in Obese Korean Patients. Yonsei Med J 2016;57:956-62. [Crossref] [PubMed]
- de Sousa AG, Cercato C, Mancini MC, et al. Obesity and obstructive sleep apnea-hypopnea syndrome. Obes Rev 2008;9:340-54. [Crossref] [PubMed]
- Bassi M, Furuya WI, Zoccal DB, et al. Control of respiratory and cardiovascular functions by leptin. Life Sci 2015;125:25-31. [Crossref] [PubMed]
- Campo A, Fruhbeck G, Zulueta JJ, et al. Hyperleptinaemia, respiratory drive and hypercapnic response in obese patients. Eur Respir J 2007;30:223-31. [Crossref] [PubMed]
- Quintas-Neves M, Preto J, Drummond M. Assessment of bariatric surgery efficacy on Obstructive Sleep Apnea (OSA). Rev Port Pneumol (2006) 2016;22:331-6. [PubMed]
- Sarkhosh K, Switzer NJ, El-Hadi M, et al. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013;23:414-23. [Crossref] [PubMed]
- Cowan DC, Livingston E. Obstructive sleep apnoea syndrome and weight loss: review. Sleep Disord 2012;2012:163296.
- Pallayova M, Steele KE, Magnuson TH, et al. Sleep apnea determines soluble TNF-alpha receptor 2 response to massive weight loss. Obes Surg 2011;21:1413-23. [Crossref] [PubMed]
- Amin R, Simakajornboon N, Szczesniak R, et al. Early improvement in obstructive sleep apnea and increase in orexin levels after bariatric surgery in adolescents and young adults. Surg Obes Relat Dis 2017;13:95-100. [PubMed]
- Ramón JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg 2012;16:1116-22. [Crossref] [PubMed]
- Dilektasli E, Dilektasli AG. Laparoscopic Sleeve Gastrectomy Improves Excessive Daytime Sleepiness and Sleep Quality 6 Months Following Surgery: A Prospective Cohort Study. Adv Ther 2016;33:774-85. [Crossref] [PubMed]
- Kohli R, Myronovych A, Tan BK, et al. Bile Acid Signaling: Mechanism for Bariatric Surgery, Cure for NASH? Dig Dis 2015;33:440-6. [Crossref] [PubMed]
- Billeter AT, Senft J, Gotthardt D, et al. Combined Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Sleeve Gastrectomy or Gastric Bypass?-a Controlled Matched Pair Study of 34 Patients. Obes Surg 2016;26:1867-74. [Crossref] [PubMed]
- Praveen Raj P, Gomes RM, Kumar S, et al. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians: "NASHOST" prospective observational trial. Surg Obes Relat Dis 2015;11:1315-22. [Crossref] [PubMed]
- Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes 2003;111:121-4. [Crossref] [PubMed]
- Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 2014;22:390-400. [Crossref] [PubMed]
- Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014;509:183-8. [Crossref] [PubMed]
- Shi X, Karmali S, Sharma AM, et al. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg 2010;20:1171-7. [Crossref] [PubMed]
- Fuks D, Verhaeghe P, Brehant O, et al. Results of laparoscopic sleeve gastrectomy: a prospective study in 135 patients with morbid obesity. Surgery 2009;145:106-13. [Crossref] [PubMed]
- Akkary E, Duffy A, Bell R. Deciphering the sleeve: technique, indications, efficacy, and safety of sleeve gastrectomy. Obes Surg 2008;18:1323-9. [Crossref] [PubMed]
- Pok EH, Lee WJ, Ser KH, et al. Laparoscopic sleeve gastrectomy in Asia: Long term outcome and revisional surgery. Asian J Surg 2016;39:21-8. [Crossref] [PubMed]
- Chiu S, Birch DW, Shi X, et al. Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review. Surg Obes Relat Dis 2011;7:510-5. [Crossref] [PubMed]
- Keidar A, Appelbaum L, Schweiger C, et al. Dilated upper sleeve can be associated with severe postoperative gastroesophageal dysmotility and reflux. Obes Surg 2010;20:140-7. [Crossref] [PubMed]
- Soricelli E, Iossa A, Casella G, et al. Sleeve gastrectomy and crural repair in obese patients with gastroesophageal reflux disease and/or hiatal hernia. Surg Obes Relat Dis 2013;9:356-61. [Crossref] [PubMed]
- Rosenthal RJ. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis 2012;8:8-19. [Crossref] [PubMed]
- Spector D, Shikora S. Neuro-modulation and bariatric surgery for type 2 diabetes mellitus. Int J Clin Pract Suppl 2010;53-8. [Crossref] [PubMed]
- Vigneshwaran B, Wahal A, Aggarwal S, et al. Impact of Sleeve Gastrectomy on Type 2 Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI 30-35.0 kg/m2: a Prospective Study. Obes Surg 2016;26:2817-23. [Crossref] [PubMed]
- Major P, Matlok M, Pedziwiatr M, et al. Changes in levels of selected incretins and appetite-controlling hormones following surgical treatment for morbid obesity. Wideochir Inne Tech Maloinwazyjne 2015;10:458-65. [Crossref] [PubMed]
- Anderson B, Switzer NJ, Almamar A, et al. The impact of laparoscopic sleeve gastrectomy on plasma ghrelin levels: a systematic review. Obes Surg 2013;23:1476-80. [Crossref] [PubMed]
- Guo X, Yin K, Chen DL, et al. Impacts of laparoscopic bariatric surgery on GLP-1 and Ghrelin level in patients with type 2 diabetes mellitus. Zhonghua Wai Ke Za Zhi 2013;51:323-7. [PubMed]
- Yousseif A, Emmanuel J, Karra E, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg 2014;24:241-52. [Crossref] [PubMed]
- Horowitz M, Edelbroek MA, Wishart JM, et al. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993;36:857-62. [Crossref] [PubMed]
- Vilsbøll T, Krarup T, Madsbad S, et al. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept 2003;114:115-21. [Crossref] [PubMed]
- Jones KL, Horowitz M, Carney BI, et al. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med 1996;37:1643-8. [PubMed]
- Ma J, Pilichiewicz AN, Feinle-Bisset C, et al. Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet Med 2012;29:604-8. [Crossref] [PubMed]
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248-256.e5. [Crossref] [PubMed]
- Huang CK, Goel R, Tai CM, et al. Novel metabolic surgery for type II diabetes mellitus: loop duodenojejunal bypass with sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech 2013;23:481-5. [Crossref] [PubMed]
- Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006;244:741-9. [Crossref] [PubMed]
- Zachariah PJ, Chen CY, Lee WJ, et al. Compared to Sleeve Gastrectomy, Duodenal-Jejunal Bypass with Sleeve Gastrectomy Gives Better Glycemic Control in T2DM Patients, with a Lower beta-Cell Response and Similar Appetite Sensations: Mixed-Meal Study. Obes Surg 2016;26:2862-72. [Crossref] [PubMed]
- Bagger JI, Knop FK, Lund A, et al. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:737-45. [Crossref] [PubMed]
- Pilichiewicz AN, Chaikomin R, Brennan IM, et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab 2007;293:E743-53. [Crossref] [PubMed]
- Huang CK, Wang MY, Das SS, et al. Laparoscopic conversion to loop duodenojejunal bypass with sleeve gastrectomy for intractable dumping syndrome after Roux-en-Y gastric bypass-two case reports. Obes Surg 2015;25:947. [Crossref] [PubMed]
- Kasama K, Tagaya N, Kanehira E, et al. Laparoscopic sleeve gastrectomy with duodenojejunal bypass: technique and preliminary results. Obes Surg 2009;19:1341-5. [Crossref] [PubMed]
- Huang CK, Tai CM, Chang PC, et al. Loop Duodenojejunal Bypass with Sleeve Gastrectomy: Comparative Study with Roux-en-Y Gastric Bypass in Type 2 Diabetic Patients with a BMI <35 kg/m(2), First Year Results. Obes Surg 2016;26:2291-301. [Crossref] [PubMed]
Cite this article as: Huang CK, Liu CC, Hsin MC, Chen YC. Sleeve and sleeve plus. Ann Laparosc Endosc Surg 2017;2:24.