Endoscopic submucosal dissection for early gastric adenocarcinoma: a review of the literature
Introduction
Gastric cancer is the fifth most common cancer worldwide, with an incidence of 952,000 new cases in 2012 (1). The highest prevalence is in the Western Pacific region, particularly China, South Korea, and Japan. Many of these countries have instituted screening programs in an attempt to reduce the high morbidity and mortality seen in this disease. Japan and Korea have national programs in which all men and women over the age of 40 undergo either upper endoscopy or barium swallow biannually; this has resulted in a documented increase in early detection of gastric malignancy (2,3). In Japan, approximately 50% of gastric cancer is now being diagnosed while still confined to the mucosa or submucosa (4).
Previously, the mainstay of treatment for early cancer was surgical resection, which is associated with a high rate of perioperative and long term morbidity despite modern techniques (5). This has prompted the development of increasingly advanced endoscopic techniques for resection of early gastric cancer. Endoscopic mucosal resection (EMR) was developed in Japan in the 1980s and was intended for complete resection of small gastric lesions confined to the mucosa. Lesions which were thought to have a low risk of lymph node or distant metastases were selected, and endoscopic treatment was performed with curative intent (6). Subsequently this technique was expanded to endoscopic submucosal dissection (ESD), which allows for deeper and more precise dissection and has led to higher rates of en bloc and complete resection even in larger lesions (7). As procedural technique has improved and specialized devices have become available, ESD is being successfully employed as treatment of increasingly complex early gastric cancer lesions.
Diagnosis
Upper endoscopy is the primary diagnostic modality for early gastric cancer. Complete evaluation of malignant lesions includes evaluation of histopathologic type, size, depth of invasion, and presence of ulceration (8). In a randomized control trial, “trimodal” endoscopy using high-resolution white light imaging in combination with optical image enhancements magnifying endoscopy-narrow band imaging and autofluorescent imaging (Olympus Medical System, Tokyo, Japan) had the highest sensitivity (89%) and specificity (100%) for the diagnosis of superficial gastric malignancies (9). These enhancements allow for analysis of surface microvasculature and have improved the diagnosis of early gastric cancer in comparison to conventional endoscopy (10). Adjuvants such as chromoendoscopy, which uses the surface application of indigo carmine dye with or without the addition of acetic acid to promote earlier washout from areas of malignancy, are widely used to delineate margins and surface structure (11). Forcep biopsy at the time of endoscopy is employed to provide pathologic confirmation of malignancy and histologic information.
Depth of invasion can be assessed using white light endoscopy based on surface characteristics. Smooth surface protrusion or depression, slight elevation of margins, and smooth tapering of folds suggests mucosal disease, while an irregular surface with marked elevation and abrupt cutting and fusion of converging folds indicates invasion into the submucosal layer (12). The role of endoscopic ultrasound (EUS) in determining mucosal and submucosal invasion in early gastric cancer is controversial. A recent meta-analysis reported a sensitivity and specificity of 76% and 72% for EUS assessment of mucosal invasion, and a sensitivity and specificity of 62% and 78% for EUS assessment of submucosal invasion (13). Another group showed that conventional endoscopy was superior to EUS for diagnosis, with an accuracy of 73.7% in the endoscopy-alone group and 67.4% in the EUS group (n=955, P<0.001) (14). In a Cochrane review of EUS for the staging of early gastric cancer, sensitivity and specificity for detection of regional lymph node metastases was also limited at 83% and 67%, respectively (15). Use of EUS is ultimately based on the preference of the endoscopist.
Resection criteria
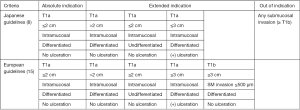
Not all early gastric cancers are amenable to resection by ESD. The Japan Gastroenterological Endoscopy Society (JGES) and the Japanese Gastric Cancer Association (JGCA) released a joint statement on guidelines for ESD in 2016 (8). A similar statement was released by the European Society of Gastrointestinal Endoscopy (ESGE) the previous year (16). These guidelines attempt to identify lesions with negligible risk of lymph node metastases. Absolute indications for ESD agreed upon by both groups are intramucosal, differentiated adenocarcinomas without ulceration and size less than 2 cm in diameter, while slight differences exist with regard to extended criteria (Figure 1).
ESD technique
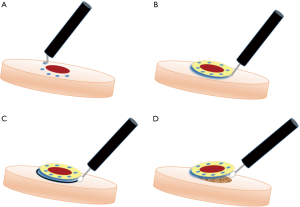
ESD can be performed under either general anesthesia or sedation. A therapeutic endoscope equipped with an energy source is used; carbon dioxide is preferred over air for insufflation due to better symptomatic tolerance (17). The procedure is performed using a standardized series of steps (Figure 2) (18). First, cautery is used to outline the circumference of the lesion to be resected. The lesion is then raised with submucosal injection of a colloidal agent which is frequently stained with indigo carmine or methylene blue dye for easy identification of the dissection plane. A specialized, insulated-tip electrosurgical knife such as the ITknife (Olympus America, Center Valley, USA) is then used to dissect first circumferentially and then deep to the lesion in the submucosal plane to achieve a complete resection. Cautery or clips are used to control any intra-procedural bleeding.
Specific training in this technique may be required to ensure adequate resection and limit complications. A Japanese training center showed that successful completion of thirty procedures was considered sufficient to achieve proficiency in easily accessible lesions in the distal stomach, with additional training required for more complex lesions or lesions located in the mid- or proximal stomach (19). Simulators including ex vivo porcine models and live pigs can be used for training prior to performance of ESD on patients (20,21). Non-human training models are particularly important for Western countries where early gastric cancer is rare.
Outcomes
ESD versus EMR
EMR is still performed in some centers for well-differentiated lesions less than 2 cm. In a cohort of 177 patients with early gastric cancer meeting criteria for both EMR and ESD, en bloc resection, complete resection, and local recurrence rates all favored ESD for lesions greater than 5 mm in size (22). In this study, there was no significant increase in complications in the ESD group. A meta-analysis published in 2014 which included the previous study and an additional nine retrospective studies comparing EMR and ESD corroborated these results, although a notably higher perforation rate (OR 4.67, 2.77–7.87) was seen with ESD (7). EMR can still be considered in selected lesions, but ESD is the mainstay for larger and more complex malignancies and allows more precise resection.
ESD versus surgery
Long term oncologic outcomes are similar between ESD and surgical resection. The longest follow-up data comes from a single Korean center in which 611 propensity matched pairs were followed after either ESD or surgical resection over ten years (23). Both absolute and extended criteria lesions were included. Overall 10-year survival was 94.9% in the surgery group and 96.7% in the ESD group (P=0.120). Complication rates varied among cohorts but tended to be lower in the groups treated with ESD (24,25). Other large series show that endoscopic complications tend to occur more frequently in the early post-procedural period, while surgical complications are more likely to occur later (23). ESD is consistently associated with shorter hospital stay than radical resection, with an average hospitalization after ESD of five to seven days versus eleven to thirteen days after surgery (23,26).
Absolute versus extended criteria
Outcomes of absolute and extended criteria ESDs are comparable. Two large Japanese studies with over 1,000 patients each showed that overall survival at five years is greater than 90% regardless of criteria used for resection (27,28). Additionally, multiple large retrospective reviews show similar results with no difference in disease free survival (28,29) or local recurrence (29,30). Most series show a statistically significant higher complete resection rate in the absolute criteria group (pooled results 91.1–95.9%) versus the extended criteria groups (pooled results 64.5–88.4%), although one multi-institutional Japanese study was able to achieve similar rates of complete resection (93.4% versus 96.4%, P=0.736) with en bloc resection >97% (P=0.867 between groups) (28-31). Local and metachronous recurrence rates did not vary between groups in any of these studies. Some groups showed higher rates of perforation (6.6% versus 2.4%, P<0.001) and bleeding (6.36% versus 3.31%, P=0.020) in the extended criteria groups (28,31). Extended criteria lesions can be technically difficult to resect, but if complete resection is achieved, oncologic results are equivalent to standard criteria lesions.
Lymph node metastases
The presence of lymph node metastases in early gastric cancer confers significantly worse survival (32), and endoscopic resection is based on the assumption that the risk of lymph node metastases is negligible. A meta-analysis of patients who had undergone gastrectomy for any T1a or T1b lesion showed an overall rate of 3.2% lymph node metastases in mucosal lesions and 19.2% in submucosal lesions (33). In seminal works in 2000 and 2001, Gotoda et al. were able to identify lymphovascular invasion, tumor size greater than 30 mm, tumor ulceration, and undifferentiated histology as independent risk factors for lymph node metastases in early gastric cancer (34,35). In a series of over 5,000 patients, when these risk factors were absent and the tumor was confined to the mucuosa or superficial submucosa (<500 µm), the incidence of lymph node metastases was zero. This forms the basis for the widely used absolute and extended criteria for resection.
Subsequent studies have validated these results by retrospective analysis of patients that had previously undergone gastrectomy and formal lymphadenectomy for early gastric cancers that would now meet criteria for endoscopic resection. Although the rate of lymph node metastases is not zero in these series, it is extremely low. A Korean study of 3,951 patients reports a rate of lymph node metastases of 0.3% in patients meeting absolute criteria and 0.4% in patients meeting extended criteria (32). Another retrospective review of over 1000 patients showed lymph node metastases in 18 (1.8%); the hazard ratio for lymph node involvement was 6.104 (95% CI: 1.1317–28.284) for undifferentiated histology (36). Refinement of criteria to accurately identify localized disease is ongoing.
Recurrence
Local and metachronous recurrences are known to occur after ESD. A Korean study showed local recurrence in 5/288 (1.6%) of ESD resections compared to 1/173 (0.6%) of gastrectomies in a propensity-matched cohort; this difference did not reach statistical significance (37). The risk of developing metachronous lesions after ESD is the main oncologic difference between endoscopic and surgical resection, with the rate of metachronous lesions ranging from 5.3% to 6.2% after ESD versus a negligible rate after gastrectomy (23,26,38). That being said, metachronous lesions can be successfully treated with repeat endoscopic dissection with no decrease in survival (26,39).
Risk factors for local recurrence in ESD have been identified. Limited studies suggest that piecemeal resections, even if negative margins are achieved, are associated with increased local recurrence (40,41). However, even following en bloc resections, local recurrence is possible. Factors associated with local recurrence include ill-defined or close (<1 mm) tumor margins and location in the upper third of the stomach. Size greater than 3 cm, lymphovascular invasion, and depth of submucosal penetration >500 µm have also been associated with positive horizontal margins and local recurrence (42,43). Differentiated histology is associated with an increased incidence of synchronous and metachronous lesions (44).
Western outcomes
Little data exists for ESD in the Western hemisphere. The only direct comparison of ESD and gastrectomy is a small Canadian study (n=30 ESD, n=37 gastrectomy) which shows no statistical difference in complete resection or two-year survival; however, these results are limited because of small sample size and inclusion of dysplastic lesions (30%) in the ESD arm (45). A German study of 91 patients demonstrated feasibility of resection of early gastric cancer and adenoma in a Western cohort but with a high failure rate and fewer en bloc resections (41). This likely correlates with the lower clinical volume and decreased training opportunities in the West.
Non-curative resection/out-of-indication lesions
Eligibility for endoscopic resection is based on preoperative assessment. A recent study showed a marked discrepancy between pre-operative and post-operative pathologic characteristics; in one series, 120/756 (15.9%) of all lesions resected by ESD were determined to be out-of-indication on final pathologic evaluations, and 29/96 (30.2%) of lesions initially classified as extended criteria were ultimately found to be out-of-indication (46). Patients found to have out-of-indication pathology are typically referred for surgery.
Efforts have been made to determine specific risk factors for recurrent tumor or lymph node metastases after non-curative resection to guide treatment. The largest series is a multicenter retrospective review from Japan in which 1,969 patients with non-curative ESD resections either underwent surgery or were followed without further intervention. A significant difference in overall survival (96.7% versus 84.0%, P<0.001) and a smaller but still significant difference in disease free survival (99.4% versus 98.7%, P=0.012) was seen between the surgery and follow-up groups at three years (47,48). Overall survival trends must be interpreted cautiously, as patients who underwent follow-up alone were significantly older with severe comorbidities. Lesions that were considered out-of-indication lesions but that did not have lymphovascular invasion or positive margins did not show increased risk of either lymph node metastases or local recurrence, suggesting that follow-up alone for carefully selected patients may be adequate (47,49).
Surveillance
Routine surveillance after ESD is important for identification of local and metachronous recurrences. Upper endoscopy after curative resections are typically performed at three to six months after the initial procedure and then subsequently every six to twelve months (8,16). Retrospective data shows a stable annual incidence of metachronous recurrence at a rate of approximately 3.5% per year that continues at least five years after initial resection even for absolute indication lesions, suggesting that long-term surveillance may be necessary (50,51). Although practiced by some groups, routine surveillance computed tomography (CT) scan is likely not necessary as the rate of detection of extragastric recurrence is extremely low; a study of 2,182 patients who underwent CT surveillance annually for five years after ESD only detected two cases of extragastric recurrence (52).
Complications
Post-procedural complications from ESD include bleeding (5%), perforation (1–9%), and stricture (1–2%). Bleeding can occur in either the early post-procedural period (<48 hours) or in a delayed fashion (≥48 hours). Multiple risk factors for bleeding have been identified including patient, tumor, and procedure characteristics. In a meta-analysis, chronic kidney disease, tumor size >2 cm, resected specimen size >3 cm, and procedure duration >60 minutes each had a statistically significant odds ratio >2.0 for bleeding risk (53). Tumor location in the lower third of the stomach has also been identified as a risk factor (54). Some groups have had success in the application of fibrin glue spray as a hemostatic agent in preventing delayed hemorrhage; one group reported a decrease in bleeding from 5.98% of patients in the control arm to 0% in the fibrin glue arm, P=0.03 (55). Antiplatelet and anticoagulation therapies are routinely discontinued peri-procedurally in most patients, although continuation of low dose aspirin does not seem to increase bleeding risk (56). Use of heparin replacement therapy and re-initiation of antithrombotic therapy are risk factors for a marked increase in delayed bleeding risk (57,58). Attempts to reduce the risk of bleeding with peri-procedural administration of proton pump inhibitors (PPIs) (59) or with routine second look endoscopy have not consistently shown a benefit (60). However, PPIs especially in combination with an agent to enhance mucosal healing such as rebamipide (approved in Japan, South Korea, and China) have shown success in healing the iatrogenic ulcerations created by ESD (61,62).
Gastric perforation is also a major source of morbidity after ESD and can occur either at the time of endoscopic resection or can present in a delayed manner in the days following resection. Overall rates of perforation from ESD range from 1.2% to as high as 9.5% (63-65). Risk factors include tumor characteristics like such as poorly differentiated histology, increased depth of invasion, location in the proximal stomach, and fibrosis of the lesion as well as patient age and length of procedure (63,65,66). Many perforations can be managed with endoscopic clipping using standard or Over The Scope Clips (Ovesco Endoscopy USA Inc., Cary, USA), suturing, and/or observation alone (64,66). More complex endoscopic techniques such as the endoloop-endoclip method have had success in perforations as large as 2 cm. In this technique, an endoloop is first clipped to healthy mucosa at the edges of the perforation. The endoloop is then tightened and secured to reapproximate the edges of the defect (67). For delayed perforation, medical management with nasogastric tube decompression and antibiotics can be trialed in the absence of peritonitis or sepsis (64). In cases of clinical decompensation or evidence of peritoneal spillage, surgical treatment with omental patch or resection is required.
Stricture is a rare complication after ESD. Large endoscopic resections leading to near circumferential ulcerations and tumors located near either the gastric cardia or antrum are at highest risk (68,69). Patients present with symptoms of dysphagia or gastric outlet obstruction. Symptomatic stenosis can be treated with balloon dilation, although gastric perforation can occur requiring further treatment or surgery (68,70). Additional treatment options include endoscopic creation of a mucosal counter-incision to relieve tension on the ESD site and intralesional or systemic steroid administration to promote granulation tissue and reduce fibrosis (69,71).
Special populations
Patients who develop gastric cancer in the setting of altered gastric anatomy from prior gastric operations or with a gastric conduit after esophageal resection present a particular challenge. ESD in these patients can be performed with adequate oncologic results in small series (72,73). In one case series of patients with a thoracic gastric conduit, pre-procedural balloon dilation of anastomotic strictures had to be performed of almost half of patients prior to passage of ESD scope, although the procedure was ultimately able to successfully proceed (72). Of note, one group reports a perforation rate of 18%, which is substantially higher than typically reported in patients with normal anatomy, due to the increased technical difficulty of ESD in these patients (74).
Conclusions
ESD is an important technique which allows for successful curative resection of early gastric cancer with low morbidity and excellent oncologic value in skilled centers. A substantial learning curve persists in low volume areas, and outcomes are worse in those areas. Further investigation is needed to establish optimal resection criteria and surveillance patterns.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.01.07). RZ serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jul 2016 to Jun 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Suh M, Song S, Cho HN, et al. Trends in Participation Rates for the National Cancer Screening Program in Korea, 2002-2012. Cancer Res Treat 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- Noguchi Y, Yoshikawa T, Tsuburaya A, et al. Is gastric carcinoma different between Japan and the United States? Cancer 2000;89:2237-46. [Crossref] [PubMed]
- Lu W, Gao J, Yang J, et al. Long-term clinical outcomes of laparoscopy-assisted distal gastrectomy versus open distal gastrectomy for early gastric cancer: A comprehensive systematic review and meta-analysis of randomized control trials. Medicine 2016;95:e3986 [Crossref] [PubMed]
- Hiki Y. Endoscopic mucosal resection (EMR) for early gastric cancer. Nippon Geka Gakkai Zasshi 1996;97:273-8. [PubMed]
- Facciorusso A, Antonino M, Di Maso M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc 2014;6:555-63. [Crossref] [PubMed]
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 2016;28:3-15. [Crossref] [PubMed]
- Kato M, Kaise M, Yonezawa J, et al. Trimodal imaging endoscopy may improve diagnostic accuracy of early gastric neoplasia: a feasibility study. Gastrointest Endosc 2009;70:899-906. [Crossref] [PubMed]
- Zhu L, Qin J, Wang J, et al. Early Gastric Cancer: Current Advances of Endoscopic Diagnosis and Treatment. Gastroenterol Res Pract 2016;2016:9638041.
- Lee BE, Kim GH, Park DY, et al. Acetic acid-indigo carmine chromoendoscopy for delineating early gastric cancers: its usefulness according to histological type. BMC Gastroenterol 2010;10:97. [Crossref] [PubMed]
- Choi J, Kim SG, Im JP, et al. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc 2011;73:917-27. [Crossref] [PubMed]
- Pei Q, Wang L, Pan J, et al. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: A meta-analysis. J Gastroenterol Hepatol 2015;30:1566-73. [Crossref] [PubMed]
- Choi J, Kim SG, Im JP, et al. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy 2010;42:705-13. [Crossref] [PubMed]
- Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev 2015;CD009944 [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Kim SY, Chung JW, Park DK, et al. Efficacy of carbon dioxide insufflation during gastric endoscopic submucosal dissection: a randomized, double-blind, controlled, prospective study. Gastrointest Endosc 2015;82:1018-24. [Crossref] [PubMed]
- ASGE Technology Committee. Endoscopic submucosal dissection. Gastrointest Endosc 2015;81:1311-25. [Crossref] [PubMed]
- Oda I, Odagaki T, Suzuki H, et al. Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc 2012;24:129-32. [Crossref] [PubMed]
- Kato M, Gromski M, Jung Y, et al. The learning curve for endoscopic submucosal dissection in an established experimental setting. Surg Endosc 2013;27:154-61. [Crossref] [PubMed]
- Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc 2014;6:112-20. [Crossref] [PubMed]
- Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009;41:746-50. [Crossref] [PubMed]
- Pyo JH, Lee H, Min BH, et al. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am J Gastroenterol 2016;111:240-9. [Crossref] [PubMed]
- Shin DW, Hwang HY, Jeon SW. Comparison of Endoscopic Submucosal Dissection and Surgery for Differentiated Type Early Gastric Cancer within the Expanded Criteria. Clin Endosc 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Fukunaga S, Nagami Y, Shiba M, et al. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc 2017;85:143-52. [Crossref] [PubMed]
- Ryu SJ, Kim B-W, Kim BG, et al. Endoscopic submucosal dissection versus surgical resection for early gastric cancer: a retrospective multicenter study on immediate and long-term outcome over 5 years. Surg Endosc 2016;30:5283-9. [Crossref] [PubMed]
- Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg 2010;97:868-71. [Crossref] [PubMed]
- Nakamura K, Honda K, Akahoshi K, et al. Suitability of the expanded indication criteria for the treatment of early gastric cancer by endoscopic submucosal dissection: Japanese multicenter large-scale retrospective analysis of short- and long-term outcomes. Scand J Gastroenterol 2015;50:413-22. [Crossref] [PubMed]
- Park CH, Shin S, Park JC, et al. Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis 2013;45:651-6. [Crossref] [PubMed]
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011;74:485-93. [Crossref] [PubMed]
- Lee H, Yun WK, Min BH, et al. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc 2011;25:1985-93. [Crossref] [PubMed]
- Choi KK, Bae JM, Kim SM, et al. The risk of lymph node metastases in 3951 surgically resected mucosal gastric cancers: implications for endoscopic resection. Gastrointest Endosc 2016;83:896-901. [Crossref] [PubMed]
- Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer 2008;11:134-48. [Crossref] [PubMed]
- Gotoda T, Sasako M, Ono H, et al. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg 2001;88:444-9. [Crossref] [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-25. [Crossref] [PubMed]
- Oh SY, Lee KG, Suh YS, et al. Lymph Node Metastasis in Mucosal Gastric Cancer: Reappraisal of Expanded Indication of Endoscopic Submucosal Dissection. Ann Surg 2017;265:137-42. [Crossref] [PubMed]
- Cho JH, Cha SW, Kim HG, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc 2016;30:3762-73. [Crossref] [PubMed]
- Kim YI, Kim YW, Choi IJ, et al. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy 2015;47:293-301. [Crossref] [PubMed]
- Choi IJ, Lee JH, Kim YI, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc 2015;81:333-41.e1. [Crossref] [PubMed]
- Horiki N, Omata F, Uemura M, et al. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc 2012;26:72-8. [Crossref] [PubMed]
- Probst A, Pommer B, Golger D, et al. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy 2010;42:1037-44. [Crossref] [PubMed]
- Fu QY, Cui Y, Li XB, et al. Relevant risk factors for positive lateral margin after en bloc endoscopic submucosal dissection for early gastric adenocarcinoma. J Dig Dis 2016;17:244-51. [Crossref] [PubMed]
- Lee JY, Cho KB, Kim ES, et al. Risk factors for local recurrence after en bloc endoscopic submucosal dissection for early gastric cancer. World J Gastrointest Endosc 2016;8:330-7. [Crossref] [PubMed]
- Park CH, Kim EH, Kang JH, et al. Low Incidence of Synchronous or Metachronous Tumors after Endoscopic Submucosal Dissection for Early Gastric Cancer with Undifferentiated Histology. PLoS One 2016;11:e0147874 [Crossref] [PubMed]
- Najmeh S, Cools-Lartigue J, Mueller C, et al. Comparing Laparoscopic to Endoscopic Resections for Early Gastric Cancer in a High Volume North American Center. J Gastrointest Surg 2016;20:1547-53. [Crossref] [PubMed]
- Kim YI, Kim HS, Kook MC, et al. Discrepancy between Clinical and Final Pathological Evaluation Findings in Early Gastric Cancer Patients Treated with Endoscopic Submucosal Dissection. J Gastric Cancer 2016;16:34-42. [Crossref] [PubMed]
- Toyokawa T, Ohira M, Tanaka H, et al. Optimal management for patients not meeting the inclusion criteria after endoscopic submucosal dissection for gastric cancer. Surg Endosc 2016;30:2404-14. [Crossref] [PubMed]
- Hatta W, Gotoda T, Oyama T, et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol 2017;52:175-84. [Crossref] [PubMed]
- Sunagawa H, Kinoshita T, Kaito A, et al. Additional surgery for non-curative resection after endoscopic submucosal dissection for gastric cancer: a retrospective analysis of 200 cases. Surg Today 2017;47:202-9. [Crossref] [PubMed]
- Min BH, Kim ER, Kim KM, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy 2015;47:784-93. [Crossref] [PubMed]
- Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 2013;62:1425-32. [Crossref] [PubMed]
- Choi KS, Kim SH, Kim SG, et al. Early Gastric Cancers: Is CT Surveillance Necessary after Curative Endoscopic Submucosal Resection for Cancers That Meet the Expanded Criteria? Radiology 2016;281:444-53. [Crossref] [PubMed]
- Libânio D, Costa MN, Pimentel-Nunes P, et al. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc 2016;84:572-86. [Crossref] [PubMed]
- Furuhata T, Kaise M, Hoteya S, et al. Postoperative bleeding after gastric endoscopic submucosal dissection in patients receiving antithrombotic therapy. Gastric Cancer 2017;20:207-14. [Crossref] [PubMed]
- Tan ES, Wang H, Lua GW, et al. Fibrin Glue Spray as a Simple and Promising Method to Prevent Bleeding after Gastric Endoscopic Submucosal Dissection. Dig Surg 2016;33:455-61. [Crossref] [PubMed]
- Matsumura T, Arai M, Maruoka D, et al. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol 2014;14:172. [Crossref] [PubMed]
- Koh R, Hirasawa K, Yahara S, et al. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc 2013;78:476-83. [Crossref] [PubMed]
- Yoshio T, Nishida T, Kawai N, et al. Gastric ESD under Heparin Replacement at High-Risk Patients of Thromboembolism Is Technically Feasible but Has a High Risk of Delayed Bleeding: Osaka University ESD Study Group. Gastroenterol Res Pract 2013;2013:365830.
- Nishizawa T, Suzuki H, Akimoto T, et al. Effects of preoperative proton pump inhibitor administration on bleeding after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. United European Gastroenterol J 2016;4:5-10. [Crossref] [PubMed]
- Mochizuki S, Uedo N, Oda I, et al. Scheduled second-look endoscopy is not recommended after endoscopic submucosal dissection for gastric neoplasms (the SAFE trial): a multicentre prospective randomised controlled non-inferiority trial. Gut 2015;64:397-405. [Crossref] [PubMed]
- Shin WG, Kim SJ, Choi MH, et al. Can rebamipide and proton pump inhibitor combination therapy promote the healing of endoscopic submucosal dissection-induced ulcers? A randomized, prospective, multicenter study. Gastrointest Endosc 2012;75:739-47. [Crossref] [PubMed]
- Kajiura S, Hosokawa A, Ueda A, et al. Effective healing of endoscopic submucosal dissection-induced ulcers by a single week of proton pump inhibitor treatment: a retrospective study. BMC Res Notes 2015;8:150. [Crossref] [PubMed]
- Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol 2012;27:907-12. [Crossref] [PubMed]
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015;21:12635-43. [Crossref] [PubMed]
- Yoo JH, Shin SJ, Lee KM, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc 2012;26:2456-64. [Crossref] [PubMed]
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228-35. [Crossref] [PubMed]
- Kato M, Uraoka T, Wada M, et al. A large muscle-layer defect of the stomach caused by endoscopic submucosal dissection is closed by using the endoloop-clips technique. Gastrointest Endosc 2016;83:1282-3. [Crossref] [PubMed]
- Iizuka H, Kakizaki S, Sohara N, et al. Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig Endosc 2010;22:282-8. [Crossref] [PubMed]
- Kim GH, Jee SR, Jang JY, et al. Stricture occurring after endoscopic submucosal dissection for esophageal and gastric tumors. Clin Endosc 2014;47:516-22. [Crossref] [PubMed]
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008;67:979-83. [Crossref] [PubMed]
- Mori H, Kobara H, Fujihara S, et al. Recanalization of severe gastric antral stricture after large endoscopic submucosal dissection: mucosal incision and local steroid injection. J Gastrointestin Liver Dis 2012;21:435-7. [PubMed]
- Mukasa M, Takedatsu H, Matsuo K, et al. Clinical characteristics and management of gastric tube cancer with endoscopic submucosal dissection. World J Gastroenterol 2015;21:919-25. [Crossref] [PubMed]
- Lee JY, Min BH, Lee JG, et al. Endoscopic Submucosal Dissection for Early Gastric Neoplasia Occurring in the Remnant Stomach after Distal Gastrectomy. Clin Endosc 2016;49:182-6. [Crossref] [PubMed]
- Nishide N, Ono H, Kakushima N, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer in remnant stomach or gastric tube. Endoscopy 2012;44:577-83. [Crossref] [PubMed]
Cite this article as: Gray KD, Moore MD, Elmously A, Zarnegar R. Endoscopic submucosal dissection for early gastric adenocarcinoma: a review of the literature. Ann Laparosc Endosc Surg 2017;2:17.