Laparoscopic resection for the treatment of hepatocellular carcinoma as a bridge for transplantation: a systematic review
Introduction
Hepatocellular cancer (HCC) represents the third leading cause of cancer-related death worldwide (1). In case of early HCC and underlying cirrhosis, liver transplantation (LT) represents the gold standard therapy, contemporaneously consenting to remove both the tumor and the whole cirrhotic liver (2). However, LT represents a scarce resource due to organ shortage. As a consequence, only very well selected patients can be successfully transplanted with an acceptable risk of post-LT recurrence: thus, neoadjuvant treatments are often required with the intent to reduce advanced tumor dimensions within transplantability criteria (downstaging approach) (3). Moreover, during the waiting list, tumor progression can be observed, with a consequent risk of patient drop-out: for avoiding it, also in this case neoadjuvant approaches can be achieved with the intent to “stabilize” tumor condition (bridge approach) (4).
Several therapies can be used with the intent to bridge or downstage HCC. Some of them present a very low rate of invasiveness (i.e., percutaneous ablation, chemo- or radioembolization) (5). In very well selected cases (i.e., small single tumors), these locoregional therapies can even be curative (6), further being able to increase post-LT survivals if performed in a multimodal fashion (7).
On the opposite, hepatic resection is a more invasive strategy, being strongly limited by several potential disadvantages, like the presence of a too impaired liver dysfunction or the risk of an insufficient post-operative future liver remnant. Moreover, when used as neoadjuvant strategy before LT, liver resection can increase LT difficulty due to the presence of multiple adhesions, potentially prolonging operative time and increasing the number of blood transfusions and the morbidity rates (8).
However, hepatic resection also presents several advantages: oncological radicality in early tumors; possibility to obtain important pre-LT data on pathological aspects of the tumor; and, possibility to delay the time-to-LT, consenting to use liver grafts for other patients (1,9,10). In this specific setting, LT is performed only after recurrence, becoming a rescue therapy named “salvage LT” (11).
Thanks to this fascinating approach, hepatic resection should not more be considered as an “alternative” but a “complementary” strategy respect to LT. Unfortunately, salvage LT is not always feasible after post-resection recurrence, due to the difficulties caused by the previous resection or by the high rate of HCC aggressiveness observable in some of these cases (12).
Starting from these considerations, the recent introduction of laparoscopic liver resection (LLR) looks to be extremely promising mainly in this specific setting. LLR is in fact connected with low post-operative morbidity rates (13), less blood loss, few adhesions and similar oncologic results in terms of R0 resection respect to open surgery (14,15).
The aim of the present study was then to perform a systematic review of the English Literature available on the specific setting of LLR and LT, with the intent to investigate the role of the laparoscopic approach in HCC patients requiring a LT.
Methods
Search strategy and screening process
A systematic search was done of the relevant studies focused on the role of laparoscopy as a bridge/DS treatment for HCC patients waiting for LT: a search of electronic databases such as MEDLINE-PubMed [1966–2016], EMBASE [1980–2016], and the Cochrane Library was conducted using standard medical subject headings (MeSH) without limits for language, gender, sample size and place of the study. We employed our search strategy in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) guidelines, as well as PRISMA for abstracts (16,17). The deadline for the date of publication was November 15, 2016.
The final MeSH terms were: (liver transplantation) AND (hepatocellular carcinoma) AND (laparoscopic resection OR laparoscopic hepatectomy).
The references of publishes articles were hand searched to find additional studies that may have been missed by the literature search.
A selection process was performed on the initially identified studies. The inclusion criteria used for this selection were: (I) articles published in English language; (II) sufficient data consisting in survival analyses (disease-free or overall survivals), ratios and 95% confidence intervals (CI) obtained by univariate or multivariate analyses, or areas under the curve and 95% CI obtained by receiver operating characteristic analyses; and (III) studies approved by local ethics committees. Exclusion criteria were: (I) studies not supplying enough statistical details; (II) review articles, nonclinical studies, letters, expert opinions, conference summaries and case reports. Moreover, only the most recent study was considered when the same patients were enrolled in more than two studies.
Data extraction
Two authors (Q Lai and J Lerut) independently reviewed the identified studies and extracted data from each study. When a disagreement occurred, the article was discussed by all the authors. The quality of the articles was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) (18): studies with scores >6 were defined as high-quality studies. For each study, the following characteristics were collected: PMID (PubMed Unique Identifier), first author’s name, reference, year of publication, country, period of enrollment, patient age, number of cases, follow-up in months and the type of statistical analyses performed in the study (Table 1).
Table 1
| doi | Author | Ref. | Year | Country | NOS | Period of enrollment | Patient age | N. cases | FU (months) | Analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| 10.1007/s00534-009-0063-0 | Laurent et al. | 20 | 2009 | France | 6 | 1998–2007 | 54.3 | 12 | 16.0 | OS, RR |
| 10.1016/j.transproceed.2013.07.014 | Casaccia et al. | 21 | 2013 | Italy | 4 | 2005–2010 | – | 3 | 48.2 | OS, RR |
| 10.1007/s13304-015-0323-2 | Felli et al. | 22 | 2015 | Italy | 4 | 2005–2014 | 54.4 | 31 | 39.2 | OS, RR |
NOS, Newcastle-Ottawa Quality Assessment Scale; FU, follow-up; OS, overall survival; RR, recurrence rate.
Results
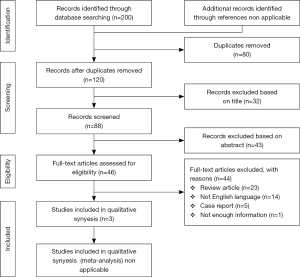
The selection process of the articles is explained in Figure 1. The various examined databases provided a total of 200 articles. After removing the duplicates, 120 articles were screened. After reading the title and the abstract, 74 articles were further removed. Of the remaining 46 papers, 43 were not considered eligible after full-text evaluation: in one case in which the investigated article matched the specific characteristics of the study, the article was however not considered for the final analysis. Despite the excluded paper presented a high number of laparoscopic cases (n=37), unfortunately no discrimination in the paper was done among open and LLR cases, thus limiting the possibility to consider this study for the intents of our analysis (19). The cohort reported in this excluded paper was also partially superimposable with the one reported in another study coming from the same centre (n=12) (20).
Indeed, only three articles effectively reported the use of LLR as a neoadjuvant strategy prior to LT, with a total number of reported cases of only 46 patients (20-22). Also considering the one previously reported discharged study, this number remained however very small (only 71 cases).
Only one study coming from France reported 12 cases of LLR compared with 12 control-group patients treated with open resection: however, despite this, the NOS of the study was only 6 (20). The other studies both coming from Italy were even less statistically solid, with no control groups and only 4 points in the NOS scale (21,22). One of these two articles reported an inhomogeneous mono-center population treated with different neoadjuvant laparoscopic approaches comprehending three cases of LLR (21). The second one was a multicenter study reporting 31 LLR patients (22).
Looking at the specific hepatic segments in which LLR was done, a total of 63 nodules were removed laparoscopically, with a prevalence in left lobe (segments 2–4: n=34; 54.0%). Unfortunately, in the study by Laurent and colleagues (20), no clarification of the site of tumor removal was done in 5 cases (7.9%). Caudate laparoscopic treatment was reported in only 2 cases (3.2%), whilst HCCs involving right lobe segments were removed in 22 cases (34.9%) (Table 2).
Table 2
| Variables | Laurent et al. (20) | Casaccia et al. (21) | Felli et al. (22) | Total |
|---|---|---|---|---|
| Site of LLR | ||||
| Segment 1 | 0 | 0 | 2 | 2 |
| Segments 2–3 | 4 | 2 | 22 | 28 |
| Segment 4 | 0 | 0 | 6 | 6 |
| Segment 5 | 0 | 0 | 8 | 8 |
| Segment 6 | 2 | 1 | 3 | 6 |
| Segment 7 | 0 | 0 | 3 | 3 |
| Segment 8 | 0 | 0 | 4 | 4 |
| Right lobe* | 1 | 0 | 0 | 1 |
| Wedge resection* | 5 | 0 | 0 | 5 |
| Total | 12 | 3 | 48** | 63 |
| Type of LLR | ||||
| Segmentectomy | 2 | 1 | *** | 3 |
| Left lateral sectionectomy | 4 | 0 | 4 | |
| Left lobectomy | 0 | 1 | 1 | |
| Right hepatectomy | 1 | 0 | 1 | |
| Wedge resection | 5 | 1 | 6 | |
| Total | 12 | 3 | 15 | |
*, no segment specified in the text; **, 48 resected nodules in a total of 31 patients; ***, no type of LLR specified in the text. LLR, laparoscopic liver resection.
Trying to look at the different types of hepatectomy performed, unfortunately only the studies by Laurent and Casaccia (20,21) clearly reported these data, whilst Felli et al. (22) only reported the segments of tumor involvement. Only looking at the first two studies, a major hepatectomy was observed in 6/15 cases (40.0%), whilst in the remaining 60.0% of cases a segmentectomy or an atypical resection were done (Table 2).
LT was performed after LLR as a salvage post-recurrence strategy in 35/46 (76.1%) cases, in 9 (19.6%) it was secondary to a bridge strategy, and in 2 (4.3%) patients it was done after cirrhosis decompensation. The mean time from resection to transplantation was only 7 months in the study by Casaccia et al. (21), while it was up to 2 years (25–29 months) in the other two studies.
Looking at the surgical aspects of LT in patients previously treated with LLR, a mean LT operative time of 6.4–7.5 hours was reported, with 1,500–2,130 mL of blood loss and a mean of 2.9–4.2 intraoperative transfusions. A clear report of adhesions in the previous site of LLR was described by the Authors in only 7/46 (15.2%) cases. Five on 46 (10.9%) patients presented a complication >3b according to the Clavien-Dindo Classification. Only one (2.2%) case of mortality was reported within a 90-day period.
Unfortunately, the study by Laurent et al. (20) only reported early post-LT course, so no definitive data are available regarding to the mid-long term results of these patients. Analyzing post-LT recurrence rate in the other two studies, after a mean follow-up of 39.2–48.2 months, only 2/34 (5.9%) recurrences were observed. Overall patient survival was 82.4% (28/34 cases), with only one (2.9%) HCC-related death.
Discussion
It is really difficult to obtain any definitive conclusion on the role of LLR prior to LT looking at the very small series reported in literature. Moreover, the only three available studies are burdened by several statistical limitations. First of all, only one study effectively identified a control group of open-resection patients, thus consenting to perform a comparison. However, such patients are retrospectively collected, and no homogeneous selection has been done among patients with regard to specific recurrence and survival confounders like tumor burden or underlying liver status. As a consequence, only a prospective study, or a propensity score trying to limit the cohort inhomogeneity should represent a possible way for obtaining acceptable statistical results.
Even worse, the other two studies only reported the laparoscopic cases, thus further limiting the impact of these studies on the possible beneficial role of mini-invasive approach respect to the open access.
It is interesting to note that all the LLR cases were finally transplanted, even in a setting of salvage LT. It is well known that non all the open resected patients finally can be transplanted: different studies reported a rate of post-resection recurred patients finally transplanted with a salvage LT ranging 26–44% (10,12,19,23). However, such a discrepancy can be easily explained looking at two important aspects: first, the laparoscopic cases have been very well selected, thus explaining the reason for a very high rate of transplantation despite a post-resection recurrence. These patients may present smaller and thus less aggressive tumors respect to a population of open resected patients. Secondly, no study has been ever designed with the intent to investigate potentially transplantable patients firstly resected with a mini-invasive approach and then transplanted after resection. If such a study has been done after open resection, no data are available after LLR, so we can only conclude that no information exists on the rate of transplantability of LLR patients after HCC recurrence.
The main problem of the fascinating approach of mini-invasive surgery as a bridge to transplantation is however connected to the fact that in 20 years from the first series of laparoscopic hepatic resection (24), less than 100 cases have been effectively reported. Such an evidence can be explained in several ways.
For example, great diffidence may exist in LT surgeons in terms of higher complexity during hepatectomy for LT due to the previous laparoscopic surgery. However, the small data reported in the present systematic review look to confirm that a mini-invasive approach minimizes the risks of adhesions, not prolonging the time of surgery during LT (19,20).
Another diffidence can derive from the fact that LLR, mainly during the learning curve, can be connected with a higher number of R1 resections. However, in the here reported series no cases of R1 resection were reported. In a large experience of 351 cases coming from 9 different French centers, only 8% of R1 margins was reported (25). A meta-analysis performed on 420 patients even reported that a wider clearance at tumor resection margins was achieved following a laparoscopic approach, with a standard mean difference of 0.34 (95% CI: 0.08–0.60; P value 0.011) (26).
The scarce attitude of LT surgeons to laparoscopy in the management of a malignant tumor harboring on a cirrhotic liver and potentially awaiting for LT may represent another explanation for these small numbers. However, over the past decade, more than 9,000 LLR procedures have been reported in the English literature (27), clearly explaining that laparoscopic resection represents a safe procedure, mainly in case of small HCC involving the left lobe.
Two consensus meetings have been recently done focusing on the role of LLR. The first one was convened in Louisville, Kentucky, US, in 2008: according to the Congress statements, LLR was proposed as a safe procedure in solitary lesions ≤5 cm in diameter, located in the peripheral liver segments (i.e., segments 2–6), with the laparoscopic left lateral sectionectomy being considered as the standard of care (28). Six years later, the second international consensus conference on LLR was held in Morioka, Japan, concluding that minor LLRs had become standard practice, whilst major liver resections still required caution (24). Interestingly enough, also the consensus conference jury underlined the fact that the available evidence coming from Literature was considered to be of low quality, thus recommending the creation of higher quality evaluative studies.
We should underline the fact that LT surgeons are diffident not only regarding to LLR, but also (perhaps even more…) regarding to open surgery before LT: a study coming from US performed on 6,817 HCC patients enlisted for LT showed that only 35 (0.5%) of them were resected before transplant, clearly showing a poor attitude of LT surgeons for using an aggressive neo-adjuvant approach (29).
Finally, a possible explanation for the low rate of LLR reported may be connected to the fact that many of these cases have not been already published: such a possibility, although improbable, is not fully impossible. It is in fact true that experience in the field of laparoscopy for malignant tumors is relatively novel, being limited in the first years by a relatively long learning curve. So, it is possible that several small monocentric series should be already published.
In conclusion, scarce evidence exists on the role of LLR before LT. In the few reported cases, laparoscopy appears to be safe, showing good results in terms of survival and post-LT recurrence. Not enough information exists on the comparative role between LLR and open resection. Further studies are surely needed with the intent to confirm the role of LLR in this specific setting.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giovanni Battista Levi Sandri) for the series “Laparoscopic Liver Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.12.11). The series “Laparoscopic Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lai Q, Avolio AW, Lerut J, et al. Recurrence of hepatocellular cancer after liver transplantation: the role of primary resection and salvage transplantation in East and West. J Hepatol 2012;57:974-9. [Crossref] [PubMed]
- Rossi M, Merli M, Lai Q, et al. Outcome after liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Transplant Proc 2007;39:1895-7. [Crossref] [PubMed]
- Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968-77. [Crossref] [PubMed]
- Cucchetti A, Cescon M, Bigonzi E, et al. Priority of candidates with hepatocellular carcinoma awaiting liver transplantation can be reduced after successful bridge therapy. Liver Transpl 2011;17:1344-54. [Crossref] [PubMed]
- Kitai S, Kudo M, Nishida N, et al. Survival Benefit of Locoregional Treatment for Hepatocellular Carcinoma with Advanced Liver Cirrhosis. Liver Cancer 2016;5:175-89. [Crossref] [PubMed]
- Lee S, Han S, Shim JH, et al. A Patient-Based Nomogram for Predicting Overall Survival after Radiofrequency Ablation for Hepatocellular Carcinoma. J Vasc Interv Radiol 2015;26:1787-94.e1. [Crossref] [PubMed]
- Ciccarelli O, Lai Q, Goffette P, et al. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int 2012;25:867-75. [Crossref] [PubMed]
- Tuci F, Vitale A, D'Amico F, et al. Survival benefit of transplantation for recurrence of hepatocellular carcinoma after liver resection. Transplant Proc 2014;46:2287-9. [Crossref] [PubMed]
- Ferrer-Fàbrega J, Forner A, Liccioni A, et al. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology 2016;63:839-49. [Crossref] [PubMed]
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132-40. [Crossref] [PubMed]
- Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology 2000;31:899-906. [Crossref] [PubMed]
- Bhangui P, Allard MA, Vibert E, et al. Salvage Versus Primary Liver Transplantation for Early Hepatocellular Carcinoma: Do Both Strategies Yield Similar Outcomes? Ann Surg 2016;264:155-63. [Crossref] [PubMed]
- Tanaka S, Takemura S, Shinkawa H, et al. Outcomes of Pure Laparoscopic versus Open Hepatic Resection for Hepatocellular Carcinoma in Cirrhotic Patients: A Case-Control Study with Propensity Score Matching. Eur Surg Res 2015;55:291-301. [Crossref] [PubMed]
- Xiang L, Li J, Chen J, et al. Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br J Surg 2016;103:1895-901. [Crossref] [PubMed]
- Cheung TT, Dai WC, Tsang SH, et al. Pure Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma in 110 Patients With Liver Cirrhosis: A Propensity Analysis at a Single Center. Ann Surg 2016;264:612-20. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for Abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med 2013;10:e1001419 [Crossref] [PubMed]
- Wells GA, Shea B, O'Connel D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Nov 15, 2016.
- Cherqui D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg 2009;250:738-46. [Crossref] [PubMed]
- Laurent A, Tayar C, Andréoletti M, et al. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2009;16:310-4. [Crossref] [PubMed]
- Casaccia M, Andorno E, Santori G, et al. Laparoscopic approach for down-staging in hepatocellular carcinoma patients who are candidates for liver transplantation. Transplant Proc 2013;45:2669-71. [Crossref] [PubMed]
- Felli E, Cillo U, Pinna AD, et al. Salvage liver transplantation after laparoscopic resection for hepatocellular carcinoma: a multicenter experience. Updates Surg 2015;67:215-22. [Crossref] [PubMed]
- Del Gaudio M, Ercolani G, Ravaioli M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant 2008;8:1177-85. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Soubrane O, Goumard C, Laurent A, et al. Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB (Oxford) 2014;16:357-65. [Crossref] [PubMed]
- Twaij A, Pucher PH, Sodergren MH, et al. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol 2014;20:8274-81. [Crossref] [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Merani S, Majno P, Kneteman NM, et al. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol 2011;55:814-9. [Crossref] [PubMed]
Cite this article as: Lai Q, Iesari S, Mennini G, Rossi M, Melandro F, Lerut J. Laparoscopic resection for the treatment of hepatocellular carcinoma as a bridge for transplantation: a systematic review. Ann Laparosc Endosc Surg 2017;2:12.