
Review: endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR)
Introduction
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) represent the progression of endoscopy from a diagnostic modality, to a useful therapeutic tool for curative intent to treat mucosal lesions and early cancers of the gastrointestinal tract. EMR is an extension of polypectomy techniques that were developed for pedunculated polyps, but applied to sessile and laterally spreading lesions. The differentiating step in EMR and ESD the procedure is submucosal injection to create a neo-polyp, or pseudopolyp, which increases tissue purchase by the hot snare. Submucosal injection as a technique was introduced in 1955 and 1973 for rigid and flexible colonoscopy (1,2). Since that time, development of EMR technique has resulted in distinct methods, categorized as injection assisted, suction cap and ligation techniques, detailed below. Piecemeal resection is possible to achieve complete resection.
To distinguish EMR from ESD, an understanding of a subtle division in layers of the gastrointestinal wall is essential. The mucosa, derived from the embryonic endoderm, and muscle arising from the embryonic mesoderm, are the two principal layers existing in the wall of the gastrointestinal tract, and are attached by a loose connective tissue submucosa (1). Both EMR and ESD involve separation of these layers at their junction in the submucosa. Histological evaluation of specimens reveals that maximal tissue depth resected is slightly greater for ESD, when compared to EMR, meaning ESD accomplishes a slightly deeper division in the submucosa (3). From a pathological standpoint, ESD then facilitates a better oncologic resection, and allows for en bloc endoscopic resections. However, the secondary implication is a higher rate of intra-procedural bleeding, as intramural blood vessels travel in this deeper layer of the submucosa. The development of ESD has, by necessity paralleled the development of electrosurgical instruments and generators to achieve coagulation, a more recent development.
Rapid adoption of ESD occurred in Asia, where incidence of gastric cancers is 8–10 fold higher than Europe and the United States. This was buoyed by national screening programs in some counties, which resulted in detection of a large number of precancerous lesions and early gastric cancers. Application of ESD then progressed to esophageal neoplasms, where early lymph node metastases are common, but where surgical resection of early cancers was excessively morbid. ESD was later applied to the colon and rectum, and this was the major driver of the introduction of ESD to Europe and the Americas.
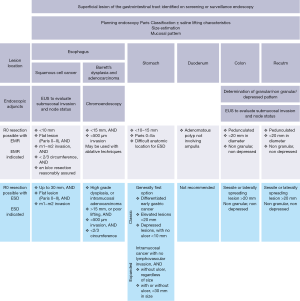
Literature evidence for EMR and ESD is stratified anatomic location of target lesions and suspected underlying etiology (see Figure 1). While there are some indications for treatment of benign lesions, the vast majority of accumulated evidence exists for premalignant and early malignant conditions. There is application for EMR and/or ESD with curative intent for metaplasia and dysplasia in of the esophagus (Barrett’s), certain early gastric cancers, and some superficial duodenal lesions that do not involve the ampulla. Few small bowel lesions beyond the duodenum can be successfully managed with endoscopic resection. Colon and rectal polyps and early cancers are also amenable to EMR and/or ESD.
Patient selection and workup
Prudent patient selection is warranted prior to attempting EMR or ESD, and is largely based on the superficial appearance of lesions within the gastrointestinal tract. The consequence of attempting EMR or ESD on an inappropriate lesion may result in incomplete oncologic resection and necessitate surgical intervention with a poorer overall prognosis. Generally EMR is indicated for nearly any benign or precancerous lesion located throughout the gastrointestinal tract. Indications for ESD are more nuanced, and are briefly reviewed below.
Esophagus
In the esophagus, where lymphatics penetrate the muscularis mucosa, earlier lymph node metastases are more common in esophageal cancer compared to other gastrointestinal malignancies. Since esophagectomy introduces a high rate of morbidity and mortality, endoscopic resection for pre-malignant and early malignant lesions is attractive, and often spares the patient an esophagectomy. For Barrett’s associated metaplasia and dysplasia, EMR is considered part of a multimodal treatment platform aimed at eradication. While complete resection of Barrett’s lesions is possible with EMR, most endoscopists use EMR techniques to remove distinct nodular areas in a background of Barrett’s. This strategy, in combination with thermal or radiofrequency ablative therapies has been shown to be safe and effective to eradicate Barrett’s lesions (4). EMR can be additionally attempted for curative intent for early moderately and well differentiated squamous cell esophageal cancer confined to the mucosa or lamina propria (5). Generally the upper limit of size for lesions amenable to EMR is at most 2–3 cm diameter and less than 1/3 the circumference of the esophageal lumen (3,4). Beyond that size, the risk of perforation, incomplete resection, and later stricture are unacceptably high. ESD is indicated for lesions of a similar size, and occupying less than 2/3 of the esophageal lumen. There is a relative indication to attempt ESD for cancers with less than 200 µm depth of invasion, as assessed by endoscopic ultrasound (6). While lesions amenable to successful R0 resection of early esophageal cancer by EMR or ESD likely differ based on the underlying phenotype (squamous cell vs. adenocarcinoma), no consensus statements that make such a distinction (7,8).
Stomach
EMR and ESD have become the mainstay of treatment for early gastric cancers in Japan, where the incidence is highest. More than 50% of gastric cancers are diagnosed at an early stage, potentially amenable to endoscopic management (6). Both ESD and EMR are safe and effective. EMR is associated with a recurrence rate of 6–10% in the reported literature, and is higher with piecemeal resection. ESD has a recurrence rate of ~1%, and is generally the preferred endoscopic technique used in Asia. ESD offers the additional advantage of clearly defining margin status and a greater chance for en bloc resection. However, the longer procedure times associated with gastric ESD compared to EMR make the latter more attractive for patients with significant co-morbid conditions. Generally accepted criteria for lesions appropriate for EMR or ESD are moderately or well differentiated adenocarcinoma and papillary carcinoma, without ulcer and less than 2 cm in diameter. Expanded criteria specify that the upper diameter limit for flat or depressed gastric lesions (Paris class IIb and IIc) is 1 cm. Because poorly differentiated adenocarcinoma and signet-ring cell carcinoma are associated with earlier lymph node involvement, these are generally not considered for EMR or ESD.
Duodenum
Duodenal adenomas occurring sporadically and not involving the ampulla can be successfully treated with EMR in some cases, with the large caveat that almost all literature for EMR in this region comes from high volume centers (9,10). Inspection with a duodenoscope is essential to adequately assess non-involvement of the ampulla. A slightly higher risk of bleeding from EMR in this region and risk of perforation likely warrants overnight observation of these patients.
Colon and rectum
EMR has its widest application in the colon and rectum, and can successfully achieve curative resection for relatively large, sessile lesions. EMR is indicated for sessile polyps 20 mm diameter or larger, with some attempting EMR up to 40mm diameter lesions. Piecemeal resection, prior intervention in the same location and difficult anatomic location are predictors of EMR failure (4). ESD generally has the same indications, but should be pursued with curative intent and careful attention to the margin status. If there is any question, referral for surgical resection is warranted.
Pre-operative preparation
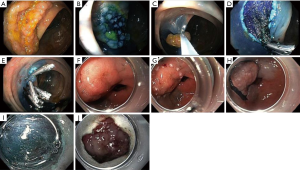
Most providers support the use of a planning endoscopy prior to attempting EMR or ESD. In addition to careful inspection, it allows the provider to adequately discuss options for management as part of the informed consent process. Visual inspection of the target lesions should occur, and be characterized by the Paris classification (11). Lesions with depressed areas and ulceration are more likely to be associated with submucosal invasion, and should be biopsied, tattooed and referred for surgical resection. A caveat exists for lesions with prior intervention, since scarring and fibrosis may be present and lesions may appear depressed. If initial visual inspection is unclear, most endoscopists evaluate saline lift characteristics (see Figure 2). Non-lifting lesions are associated with deeper invasion and should be referred for surgical resection. Magnified views of surface architecture and pattern of crypts, both with and without augmentation with topical dyes are commonplace in Asia, but are not in wide use in the West, mostly due to the unavailability of magnifying endoscopes. Other adjuncts such as endoscopic ultrasound may further aid in determining appropriate lesions for endoscopic resection. Tattooing can be helpful, but should not be placed within the target lesion, as it can cause tissue fibrosis.
Planning for the approach to sedation and anesthesia and management of co-morbid conditions is beneficial. In the United States, most endoscopic procedures are performed using a combination of benzodiazepine and narcotic, such as midazolam and fentanyl, to achieve moderate sedation. Propofol may also be appropriate, though we qualify that statement with the reality that it is not available to non-anesthesiologists in all practice areas, and does require additional training and patient monitoring devices (12). Either technique is likely appropriate for EMR; however, long procedure times sometimes associated with ESD often make general anesthesia a better option. Cardiac and respiratory comorbidities should be evaluated prior to pursing EMR or ESD, especially if a patient is to undergo general anesthesia. Bleeding is one of the major complications of EMR and ESD, and patients on chronic anticoagulation and antiplatelet agents may be at higher risk. Standard protocols for peri-procedural management of anticoagulation and antiplatelet agents should be followed (13).
Adequate bowel preparation is essential for lesion detection and visualization during resection. For esophageal and gastric lesions, a clear liquid diet for 1 day prior to the procedure and then nil per os for 6 hours prior to the procedure is typically sufficient, barring a functional disorder of the esophagus or stomach. For colon and rectal lesions, polyethylene glycol preparation is preferred. If inadequate preparation is encountered, EMR or ESD should not be attempted. A secondary preparation should be administered and the patient rescheduled for the following day.
In addition to patient evaluation, preparation and optimization, consideration must be made to the preparation of the provider. ESD has been slowly adopted in the West, and a relative paucity of advanced endoscopists have the requisite skills or experience. Lastly, reflecting the multidisciplinary management paradigm surrounding treatment of most malignant conditions, relationships with thoracic, general and colorectal surgeons and medical oncologists should be well established.
Equipment preference card
- Therapeutic panendoscope with video monitor and lavage system. Video recording capabilities are useful when learning EMR or ESD.
- Electrosurgical generator capable of implementing customized settings for EMR and ESD. Our institution uses Erbe Vio® 300D (Erbe USA, Marietta, Georgia, USA) equipped with both water jet and argon plasma coagulator (APC) modules and a foot pedal.
- Carbon dioxide insufflation system.
- For EMR.
- iSnare System® (US Endoscopy, Mentor, Ohio, USA).
- For the esophagus, Duette® multi band mucosectomy kit is used (Cook Medical, Bloomington, Indiana, USA).
- Roth Net® (US Endoscopy, Mentor, Ohio, USA).
- A variety of hemoclips are used.
- For ESD.
- A variety of injection needles and injection solutions are used.
- Both straight and oblique caps may be used.
- APC™ 2 (Erbe USA, Marietta, Georgia, USA).
- DualKnife™ (Olympus Endoscopy, Central Valley, Pennsylvania, USA).
- ITknife nano™ (Olympus Endoscopy, Central Valley, Pennsylvania, USA).
- A variety of hemoclips are used.
Procedure
EMR or ESD may take place in a dedicated endoscopy suite. While EMR can often be performed under moderate sedation with benzodiazepine with narcotic sedation, longer procedure times associated with ESD often make general anesthesia a better option. There is a low incidence of bacteremia after EMR, and thus most providers do not administer perioperative antibiotics (14). Once a lesion is located, stable access and visualization is paramount. There has been considerable work done regarding settings and pulse wave patterns of electrosurgical units in combination with various electrosurgical generators, and is outside the scope of this review (15).
EMR
Regardless of the specific technique, the first two steps for EMR are to locate the lesion, and perform a submucosal injection. Injection is accomplished with a 21–25 G needle to create a neo-polyp (see Figure 2). Multiple injections may be necessary to achieve a relatively uniform lift. There are numerous injection solutions available depending upon practice location. Small case control studies of head to head comparisons exist, but choice of solution is largely based on individual preference (10,16). Generally more viscous solutions produce more durable lifts. Addition of indigo carmine or methylene blue can aid in the identification of the submucosal layer, and more importantly the lateral margin of target lesions. Resection of the lesion can often be accomplished with a single resection, but piecemeal resection is possible if lesions are larger. Resection occurs by one of the following techniques:
Snare
A hot snare that is sized to be slightly larger than the target lesion is introduced through the working channel of the endoscope. The submucosal injection generally allows for greater tissue purchase on the far edge of the lesions. The lesion is encircled, and the snare is set, ensuring that there is not too much bunching of the tissue. Application of the electrocautery frees the lesion. This can be serially repeated if necessary for piecemeal resection.
Suction or cap assisted EMR
If this technique is to be used, a clear plastic cap is applied to the end of the endoscope. The submucosal injection is performed with the cap in place. Apposing the cap to the tissue, while applying suction creates a neo-polyp within the cap. A hot snare is then introduced, and generally falls along the circumference of the cap. Following application of electrocautery, the lesion can often be removed with the scope if the suction is maintained. For small lesions a trap should be placed in the suction tubing. The suction cap technique is most useful in the stomach and esophagus.
Band ligation
If this technique is to be used, a clear plastic cap with a band ligation system is applied to the end of the endoscope. The submucosal injection is performed with the cap in place. Application of suction again elevates tissue into the cap. The band is then deployed. A hot snare is then introduced through the instrument port, and used to resect the neo-polyp. The snare may be applied below, across, or above the band. There are some providers who do not perform a submucosal injection prior to application of this technique.
ESD
ESD begins similarly to EMR, with lesion location. It is also important to ensure that visualization of the lesion is adequate prior to embarking on the next steps. In certain situations, lesions are more accessible in a retroflexed view, in which case a pediatric diameter endoscope can be useful, however, the decreased rigidity of a narrow caliber endoscope can make resection challenging in other ways. Defining the lateral margin of lesions is perhaps the most important step, as ESD is typically undertaken for curative intent (see Figure 2). Use of surface dyes, chromoendoscopy, narrow band imaging, magnification or other adjuncts cannot be understated, but is outside the scope of this review. Circumferential mucosal marking is the next step, and accomplished with a needle knife or APC. Marks are made roughly 5 mm outside the lateral margin of a gastric lesion. For esophageal adenocarcinoma marking is 5–10 mm outside, or at the margin for squamous cell carcinoma. In the colon, lesion borders are often more obvious, and marking may not be necessary. A circumferential mucosal incision is made just outside the mucosal markings. A submucosal injection is then performed with a 21–25 G needle to lift the lesion. Submucosal dissection is then performed with the assistance of a cap and an ESD specific knife. The distal tip of the cap elevates the lesion and an electrosurgical knife dissects parallel to the muscular layer. Any bleeding encountered should be controlled expeditiously, as blood can quickly and easily obscure the dissection plane. Submucosal dissection is continued until the lesion is completely free. Small lesions can often be extracted within the application of suction through the cap, while a Roth net may be necessary to retrieve larger lesions. Generally orienting the lesions to the 5 o’clock position is advantageous, and allows the best combination of scope maneuverability and visualization. If possible, positioning the patient such that lesions is in an anti-dependent position allows gravity to aid in retraction (3).
Post-operative management
As with most endoscopic procedures, most patients are able to return home after the procedure. Hospitalization is the exception rather than the rule. Nearly all EMR procedures can be performed as ambulatory procedures. ESD carries a higher risk of bleeding complications compared to EMR, and as such, may warrant overnight observation. This is especially true for providers that are embarking to establish a new ESD practice.
In the immediate post-operative period, monitoring focuses on detecting early complications with either EMR or ESD. Bleeding is the most common complication. Bleeding occurring after EMR is rare in the esophagus, but occurs in an estimated 0–11% of gastric lesions and 2–22% of colonic lesions (10). Most bleeding events occur during the index procedure, and the vast majority of post-procedural bleeding presents within 24 hours; however delayed bleeding may occur up to 5% of the time (10). Prophylactic coagulation of visible non bleeding vessels has not been shown to decrease bleeding after EMR. Bleeding after ESD is also common, occurring in 4.5–15.6% of cases, and this risk increases with lesion size (17). Judicious intraprocedural hemostasis is thus encouraged. For gastric and duodenal lesions, proton pump inhibitor and mucosal protectant medications are associated with decreased bleeding rates. Perforation after EMR and ESD is thankfully uncommon. EMR associated perforations occur 0.5–1% of the time, with some variation based on location, reflecting differences in thickness of the gastrointestinal wall. ESD has higher perforation rate of 2–4%. ESD-related perforations do tend to be smaller, due to the small size of the knives used. EMR related perforations can be quite large, as they result from the muscularis mucosa being gathered into the snare. Management of perforation can range from endoscopic management with clips and suturing if recognized during the index procedure, to percutaneous drainage, or surgical exploration of the abdominal or thoracic cavity.
Following EMR or ESD, surveillance endoscopies are warranted to monitor for recurrence, de novo lesions, and to evaluate for strictures. There is no consensus on the time interval for surveillance endoscopy, but an initial diagnostic endoscopy at 3–6 months post resection is reasonable in most cases. EMR and ESD related strictures are most common in the esophagus, affecting 12–17% of patients (10,17). These can often be palliated with pneumatic balloon dilation. Unfortunately, recalcitrant strictures may require operative intervention.
Tips, tricks and pitfalls
The learning curve for ESD is substantial, though it does depend on the background of the individual endoscopist. In Asian training paradigms, independent proficiency for ESD has been shown after 30–40 ESD procedures. Procedural outcomes become similar to experts after >80 procedures (18-20). For this reason, training is likely best performed in tertiary centers, where a concentration of patients with amenable lesions are likely to exist.
Several authors have proposed training paradigms for endoscopists outside of Asia to obtain the requisite skills to be credentialed to safely perform ESD (6,21). These authors advocate for travel to high volume Asian centers for initial observation and training. This should be followed by dedicated training time in both ex vivo and in vivo animal models, which are typically pigs or dogs. Initial attempts at ESD should be proctored by an endoscopist experienced in ESD. While capabilities for video-based mentoring are increasing internationally, this is likely not appropriate for the initial ESD attempts, and is better delayed until the endoscopist has completed at least a few ESD procedures with an immediately present proctor (22).
Authors have also noted that ability to performed ESD varies by anatomic location. Distal gastric lesions are technically easier, followed by proximal gastric lesions. The decreased incidence of these lesions in the West, but commonality of colonic lesion makes the latter the most attractive entry point for Western providers to perform ESD (6).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.11.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenberg N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg 1955;70:120-2. [Crossref] [PubMed]
- Deyhle P, Jenny S, Fumagalli I. Endoscopic polypectomy in the proximal colon. A diagnostic, therapeutic (and preventive?) intervention. Dtsch Med Wochenschr 1973;98:219-20. [Crossref] [PubMed]
- Saunders BP, Tsiamoulos ZP. Endoscopic mucosal resection and endoscopic submucosal dissection of large colonic polyps. Nat Rev Gastroenterol Hepatol 2016;13:486-96. [Crossref] [PubMed]
- Chandrasekhara V, Ginsberg GG. Endoscopic mucosal resection: not your father's polypectomy anymore. Gastroenterology 2011;141:42-9. [Crossref] [PubMed]
- Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery 1998;123:432-9. [Crossref] [PubMed]
- Bhatt A, Abe S, Kumaravel A, et al. Indications and Techniques for Endoscopic Submucosal Dissection. Am J Gastroenterol 2015;110:784-91. [Crossref] [PubMed]
- Huntington JT, Walker JP, Meara MP, et al. Endoscopic mucosal resection for staging and treatment of early esophageal carcinoma: a single institution experience. Surg Endosc 2015;29:2121-5. [Crossref] [PubMed]
- Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high-grade dysplasia versus intramucosal carcinoma in Barrett's esophagus. Gastrointest Endosc 2015;81:1158-66.e1-4.
- Kedia P, Brensinger C, Ginsberg G. Endoscopic predictors of successful endoluminal eradication in sporadic duodenal adenomas and its acute complications. Gastrointest Endosc 2010;72:1297-301. [Crossref] [PubMed]
- ASGE Technology Committee. Endoscopic mucosal resection. Gastrointest Endosc 2015;82:215-26. [Crossref] [PubMed]
- Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570-8. [Crossref] [PubMed]
- Vargo JJ, Cohen LB, Rex DK, et al. Position statement: nonanesthesiologist administration of propofol for GI endoscopy. Gastrointest Endosc 2009;70:1053-9. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3-16. [Crossref] [PubMed]
- Lee TH, Hsueh PR, Yeh WC, et al. Low frequency of bacteremia after endoscopic mucosal resection. Gastrointest Endosc 2000;52:223-5. [Crossref] [PubMed]
- ASGE Technology Committee. Electrosurgical generators. Gastrointest Endosc 2013;78:197-208. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kashimura K, et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy 2004;36:579-83. [Crossref] [PubMed]
- ASGE Technology Committee. Endoscopic submucosal dissection. Gastrointest Endosc 2015;81:1311-25. [Crossref] [PubMed]
- Gotoda T, Friedland S, Hamanaka H, et al. A learning curve for advanced endoscopic resection. Gastrointest Endosc 2005;62:866-7. [Crossref] [PubMed]
- Oda I, Odagaki T, Suzuki H, et al. Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc 2012;24:129-32. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Draganov PV, Gotoda T, Chavalitdhamrong D, et al. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013;78:677-88. [Crossref] [PubMed]
- Bhatt A, Abe S, Kumaravel A, et al. Video-based supervision for training of endoscopic submucosal dissection. Endoscopy 2016;48:711-6. [Crossref] [PubMed]
Cite this article as: Strong AT, Ponsky JL. Review: endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR). Ann Laparosc Endosc Surg 2016;1:44.


