Prevention and management of hemorrhage during a laparoscopic colorectal surgery
Introduction
Laparoscopic techniques have been widely used in the surgical treatment of gastrointestinal tumors. In particular, the feasibility of laparoscopic surgery for colorectal cancer has been well documented in a number of prospective, randomized, and controlled clinical studies. Among them, the laparoscopic radical resection of colorectal cancer has been recognized by the US National Comprehensive Cancer Network (NCCN) as an optional therapy. In fact, the laparoscopic radical operation for rectal cancer has an obvious advantage of being minimally invasive. While achieving minimal invasive and radical resection are the main targets of surgeries for tumors, safety is the foundation and prerequisite of a successful surgery. For beginners, performing a laparoscopic colorectal surgery in a safe and reliable way is the only way to success.
One of the most important and notable surgical complications is intraoperative hemorrhage. In particular, severe bleeding is one of the major complications of some laparoscopic colorectal surgeries and also one of the major causes of conversion to laparotomy.
Severe intraoperative bleeding is often caused by the injury of major blood vessels. The main causes of intraoperative hemorrhage include: (I) inadequate knowledge of the anatomical courses of the vessels under laparoscope; (II) poor identification of anatomic layers; (III) lack of correct and effective traction and effective exposure of visual field; and (IV) lack of cooperation among skillful team members. During the surgery, the operator must be able to identify the anatomic courses of vessels under the laparoscopic view and carry out correct and effective traction, so as to effectively expose the surgical field, obtain good surgical view, and thus prevent any possible injury of any vessel. This proposes higher requirements on the operator, the first assistant, and the camera holder, especially close cooperation and good teamwork. The operator must perform separation and dissection in correct anatomic layers to avoid vascular injury and bleeding.
As a young surgeon who is now able to independently carry out laparoscopic radical surgery for colorectal cancer, I had some experience in encountering intraoperative hemorrhage and also have seen or heard much from my mentors and peers, which I would like to share in this article.
Prevention of hemorrhage in a laparoscopic colorectal surgery
Identifying the appropriate anatomical landmarks
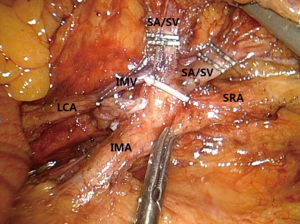
Surgeries in rectum or left colon: during the laparoscopic radical treatment of rectal cancer, left colon cancer, and sigmoid colon cancer, the vessels (mainly inferior mesenteric vessels and their branches) to be handled during the surgery are relatively simple. The inferior mesenteric artery (IMA) originates at the midway between the abdominal aorta at the level of the duodenum and the aortic bifurcation and then courses towards the left lower side, showing slight bulge and beating. The inferior mesenteric vein (IMV) is purple in color under laparoscope; it leaves the IMA and arises alone, with its medial landmark being the ascending part of the duodenum and the peritoneal folds of the duodenum and jejunum and the head-side landmark being the lower edge of the pancreas. A key skill for laparoscopic exposure of IMA and IMV is as follows: the assistant pulls the inferior mesenteric vascular pedicles towards the left upper side vertically, so as to form a triangle operating window with the abdominal aorta. By doing so, the unnecessary vascular injury and intra-abdominal hemorrhage can be avoided. During the dissection of the inferior mesenteric vessels, the operator uses a HIFU to complete technical actions such as picking, pulling, isolating, and separating, so as to skeletonize the vessels. This enables both lymph node dissection and prevention of bleeding. Meanwhile, the direction of HIFU energy surface should be carefully adjusted to avoid any vascular injury or bleeding. Meanwhile, during the division of the IMA root, the relationship between IMA and IMV and that between IMA and its branches [including left colic artery (LCA) and sigmoid colon artery] should be taken into consideration. In general, when IMA is closer to the root, it has a longer distance away from IMV. Therefore, when IMA is transected at its root, it will be less likely to injure IMV, left colon vessels, or sigmoid vessels. During the left colon vessel-preserving D3 radical treatment for rectal cancer or the superior rectal artery (SRA)-preserving D3 radical treatment for left colon cancer, a good knowledge of this anatomic feature will be particularly useful for the protection of vessels (Figure 1).
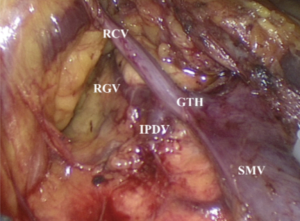
Right colon surgeries: the anatomic layers encountered during a right colon surgery are far more complex than those for left colon, rectum, and sigmoid colon; meanwhile, there are more anatomic variations in the arteries of the right colon. In fact, surgeries involving right colon are featured by a large number of variations. The right colon vessels, in particular the vein, have relatively more anatomic variations. While the ileocolic vein has a relatively fixed position and course, the right colic vein, middle colic vein, and gastrocolic common trunk often have variations. It has been reported that the frequencies of occurrence of the gastrocolic trunk and middle colic vein were 69% and 85%, respectively, whereas the frequency of the absence of right colon vein could be up to 57%; furthermore, the frequency of the right colon artery ranges only 20–30%. The branches of gastrocolic trunk also have many variations (Figure 2), which include the typical three-branch type (i.e., right colic vein, right gastroepiploic vein, and anterior superior pancreaticoduodenal vein), two-branch type (right colic vein and right gastroepiploic vein), and even variation that the right colic vein directly connects the superior mesenteric vein (SMV). The presence of these variations dramatically increases the risk of bleeding during a laparoscopic surgery for right colon lesions. In particular, during the handling of the common trunk, since this vessel has short and thick anatomic structures and crispy texture, excessive traction may tear the common trunk and thus lead to bleeding; even worse, improper management can cause fatal bleeding of SMV; in contrast, inadequate traction can also result in poor exposure of anatomic layers, thus leading to bleeding. Therefore, at the beginning of the surgery, it’s better to use ileocolic vascular pedicles as the anatomic landmarks; then, divide the colonic mesentery at its lower edge to open a window, which enables the operator to further search for the right retrocolic space (RRCS) and then trace and dissect the SMV along the ileocolic vessels. SMV is the main line during the surgical anatomy of the whole right colon. Dissect mesenteric adipose tissue on its surfcace to completely expose SMV; along the SMV, the dissection moves upwards to expose the branches of the arteries and veins at its right side. The ligation and transection of these branches not only are less likely to cause bleeding and meanwhile ensure the thorough D3 lymph node dissection. The same skill can be applied during searching for the common trunk and its branches, so as to adequately dissect and expose these vessels. The common trunk is often located within the range from the lower edge of pancreatic head to the line 2 cm upper from the lower edge of pancreatic head. Thus, the lower edge of the pancreatic head can also be used as one of the landmarks during searching for common trunk in a laparoscopic right colon surgery.
Entering the correct anatomical layers
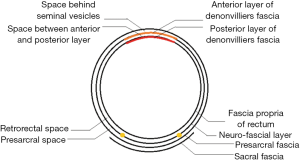
Along with research advances in the local anatomy of the colorectal regional under laparoscope, it has been well documented that the Toldt’s space is a vessel-free space located between mesorectum/mesocolon and prerenal fascia (PRF). Due to the continuity of PRF, it is a mutually intersecting anatomic layer that can be used as a surgical plane during the colorectal surgery. There are only two substantive obstacles: the inferior mesenteric vessels and the lateral ligaments of rectum (may include middle rectal artery). For the rectum, this plane is initially seen as the retrorectal space, which extends towards its tail side till the S4 level, at which the mesorectum and the neuro-fascial layer merge into the rectosacral fascia (Waldeyer fascia). During the operation this fascia must be divided before entering the presacral space behind it (Figure 3). For the left colon, this plane extends upwards to form the left retrocolic space and the transverse retrocolic space (TRCS), till the lower edge of the body and tail of the pancreas; in the right colon, it is seen as the RRCS and the TRCS. In addition, the intermembrane space (IMS) between the posterior layer of the great omentum and the top of the transverse mesocolon can communicate with the RRCS via the posterior side of the root of transverse mesocolon; therefore, IMS must be entered during the dissociation of transverse mesocolon in a left/right colon surgery. In addition, IMS can be entered by extending towards the head side in the RRCS/left retrocolic space, so as to completely resect the transverse mesocolon (Figure 4).
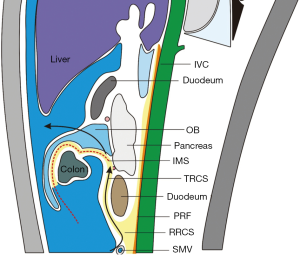
In the clinical settings, a medial approach is recommended during the entering of the proper anatomic layers. During a rectal surgery, the assistant lifts the inferior mesenteric vascular pedicle and the mesenteries of rectum and sigmoid colon towards the abdominal side and meanwhile expand and tighten the mesenteries, thus producing adequate tension. Thus, with the sacral promontory as the starting landmark, the operator cut open the mesenteries towards the head side. Due to the atomization effect of HIFU, the space behind the rectum and the sigmoid colon naturally appears. Then, toward the tail side the retrorectal space can be gradually extended, and then the rectal fascia can be broken through to reach the presacral space; towards the head side the root of the IMA can be reached for further lymph node dissection. In this correct layer, it can be found that the proper rectal fascia wraps the fat inside the mesorectum and gently hangs down, which is smooth and thin under the endoscopic light source. After the inferior mesenteric vessels are handled, the dissection continues towards the head side till the space behind the left colon. Thus, during the left colon surgery, the same strategy can be applied to find the proper space behind left colon, so as to avoid that the application of lateral approach may lead to the entering of the excessively deep layer or even the posterior side of left kidney. Similarly, the medial approach can also be applied during a right colon surgery. As described above, the mesenterium can be cut open at the lower edge of the ileocolic vascular pedicles; then, the RRCS is entered and extended towards the head side. The duodenum and pancreatic head can be used as landmarks successively to make sure that the right space has been extended. Similarly, it can be found that the posterior lobe of the right mesocolon also holds the fat inside the mesentery and gently hangs down, which is smooth and thin. The dissection continues towards the head side to reach the TRCS and the IMS, so as to finalize the dissociation of transverse mesocolon. Towards the lateral side it can reach the peritoneum.
In summary, both sharp and blunt separation can be carried out in such a proper vessel-free surgical plance, which is a key strategy for preventing severe bleeding in abdominal or presacral cavity during a surgery.
Management of hemorrhage in a laparoscopic colorectal surgery
If severe bleeding occurs during operation, the bleeding site should be controlled as soon as possible. In this process, the operator must keep calm; meanwhile, it is equally important that the assistant and camera holder provides calm response and cooperation. Severe bleeding often has a serious impact on the laparoscopic vision, including brightness and sharpness. The “eyes” of the operator depends on the actions of the camera holder. Often a camera holder is a doctor with lowest qualification; when bleeding occurs, his performance has important impact on the operator’s operations in stop bleeding. Thus, teamwork is particularly important. The camera holder must be trained to know how to avoid the impact of pulsatile bleeding on the camera, how to keep the vision clear, and how to put the main surgical field at the center of the camera. The assistant shall thoroughly expose the surgical field by adequate traction and/or effective suctioning, so that the operator can use the left hand to cooperate with the right hand to stop bleeding. For bleeding caused by vascular injury, under the active cooperation of the assistant, the operator can use the instrument in left hand to oppress or clamp the vessel firstly; then, based on the diameter of damaged blood vessel, electro-coagulation, titanium clamps, HIFU, Ligasure, absorbable clips, or large plastic clips may be applied for hemostasis; if necessary, suture ligation may be applied for hemostasis. Same as hemostasis in conventional surgeries, clamping without clear visual field should be avoided; instead, it should be performed after the bleeding has been controlled. If condition allows, the bleeding vessel can be adequately dissociated and exposed before clamping, so as to leave sufficient space for operation and thus achieve proper hemostasis. Remember: once bleeding occurs during surgery, there are limited chances for successful hemostasis. Thus, bleeding should be effectively stopped during each operation. For oozing blood (e.g., presacral hemorrhage), the bleeding may be controlled by compression firstly, followed by sunctioning of the remained blood and clearing of the visual field; finally, hemostatic gauze or bone screw may be applied for hemostasis. In most cases, hemostasis can be completed under laparoscope. Injury of a large blood vessel may lead to massive bleeding in the abdominal cavity; in such cases, conversion to celiotomy should be arranged immediately. Blind pursuit of laparoscopic surgery may miss the chances of hemostasis and therefore should be avoided.
Prevention and treatment of anastomotic bleeding
Anastomotic bleeding during a colorectal surgery (especially the surgeries for rectal cancer) is one of the severe compliations early after surgery. In most cases it occurs after surgeries for low rectal cancer. In fact, the anastomotic bleeding often occurs immediately after intraoperative anastomotic operation; however, since the bleeding is obscure, the condition will not be found until the patient is transfered to the ward. According to our experience, for patients with low rectal cancer, the intestinal canal at the distal end of the tumor shall be skeletonized as thoroughly as possible before it is transected. When a cutter & stapler or a circular stapler is applied, the stapling shall be maintained for about 15–20 s to ensure the sufficient compression and closure of the vessels inside the tissue before the transection of the intestinal canal, which can minimize the potential risk of bleeding.
Furthermore, after the anastomotic operation is completed, the intraoperative endoscopic examination is clinically meaningful for the assessment of anastomosis: it enables the early detection and timely management of anastomotic bleeding and proactively eliminates any potential bleeding, thus minimizing the risk of postoperative anastomotic bleeding. According to our experience, after the anastomotic operation is completed, the condition around the anastomosis should be carefully observed during the intraoperative endoscopic examination. If anastomotic bleeding is found, the accumulated blood should be suctioned off firstly; then, appropriate management is provided, which includes intraoperative endoscopic hemostasis using titanium clips or electrocautery unit; alternatively, suturing under direct vision may be performed to stop bleeding in the ultra-low anastomosis, so as to reduce the occurrence of postoperative anastomotic bleeding. Any active bleeding must be handled immediately; delayed treatment may lead to postoperative bleeding, which might require a second hemostasis treatment.
The management strategy of postoperative anastomotic bleeding is as follows: for minor hemorrhage, conservative treatment is preferred, along with the close observation of the bleeding volume and the patient’s general condition; for major hemorrhage or in patients with unstable hemodynamics or in patients who are unresponsive to conservative treatment, non-conservative treatment should be applied. There are three specific endoscopic hemostatic therapies: spraying a haemostatic agent; electrocoagulation; and use of hemostatic clips. The endoscopic hemostatic approaches have many advantages: direct vision of the bleeding site; assessment of bleeding volume; small trauma; fewer complications; and avoiding prolonged hospital stay and saving treatment costs due to a second surgery. Many surgeons also believed that endoscopic techniques are safe and effective in treating anastomotic bleeding after a surgery for rectal carcinoma and proposed that an endoscopic hemostatic approach is preferred before a second hemostatic surgery.
When endoscopic hemostasis is applied, the patient’s general condition should be carefully observed to ensure that the operation can be performed as quickly as possible when the vein is cut open and fluid replacement is performed for volume maintenance. During the operation, the accumulated blood and blood clots in the intestinal cavity should be suctioned off to improve the visual field inside the intestinal cavity; then, the bleeding site should be carefully searched for to allow appropriate hemostatic treatment. A small amount of oozing blood may exist at the anastomosis; in such cases, endoscopic spraying of epinephrine may be performed to stop bleeding; alternatively, an electro-coagulation hemostasis method may be applied, during which thermal coagulation may contribute to tissue edema and compress the blood vessels, thus achieving the hemostatic effect. For a larger amount of oozing blood or pulsatile bleeding, spraying of adrenaline should be followed by clamping with titanium clip, particularly for bleeding from a naked blood vessel or a small artery.
For surgeons, while surgical complications can not be completely avoided, there are still chances for reducing the incidence. Intraoperative hemorrhage can be caused by a variety of factors, among which anatomy, operations, and teamwork are most important. Laparoscopic colorectal surgeons must have solid knowledge on the key laparoscopic anatomic landmarks of colon and rectum and the natural anatomic layers under laparoscope and meanwhile grasp the basic operational skills required in a laparoscopic colorectal surgery. Furthermore, the surgeons should also be familiar with the prevention and management of various complications, including hemorrhage. During this process, the operator should never forget that teamwork is equally important in performing a successful laparoscopic colorectal surgery. It is for sure that the quality of laparoscopic colorectal surgeries will be constantly improved and the complications will be reduced if we strictly follow the surgical specifications, introduce new techniques in a balanced way, and continue to strengthen the technical training and learning.
Conclusions
Intraoperative hemorrhage is a common complication in colorectal surgery that needed to be identified and managed as soon as possible. It requires the surgeon to have a sound comprehension of the anatomy of colon and rectum, qualified laparoscopic operation skills, a calm mind and good teamwork.
Acknowledgments
Funding: Supported by National Natural Science Foundation of China (NFSC: 81572818).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.11.22). Ma J serves as the Managing Editor of Annals of Laparoscopic and Endoscopic Surgery. Zheng M serves as the Editor-in-Chief of Annals of Laparoscopic and Endoscopic Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Cite this article as: Zhang L, Ma J, Zang L, Dong F, Lu A, Feng B, He Z, Hong H, Zheng M. Prevention and management of hemorrhage during a laparoscopic colorectal surgery. Ann Laparosc Endosc Surg 2016;1:40.