Novel metabolic surgery: first Asia series and short-term results of laparoscopic proximal jejunal bypass with sleeve gastrectomy
Introduction
With the advent of rapid urbanization, shift if diet preferences and sweeping socio-cultural changes in Asia where the majority of the world population resides, obesity and its associated metabolic consequences have risen to alarming proportions (1-3). Asians, in general, are different phenotypically from Caucasians and it has been recognized that Asians are genetically prone for metabolic syndrome, adiposity and are at a heightened risk for cardio-vascular mortality (3-5).
Though bariatric surgery has evolved through the years, there is a scope for further refinement as our knowledge and understanding of the processes that lead to and sustain obesity grows. Newer surgeries and innovations need to be designed that incorporates this knowledge and which can be shown to be equally or more effective while minimizing the short and long term adverse effects seen in the currently performed obesity surgeries (6). Patients with obesity come in a very wide range of BMIs and varying degree of co-morbidities. Instead of a few routine surgeries applied to all patients, what would be ideal is a surgery tailored to the patients uniquely altered physiology that would give the best possible results while minimizing the adverse effects. Hence, a wider repertoire of surgical options need to be created.
Laparoscopic proximal jejunal bypass with sleeve gastrectomy (LPJB-SG) was developed in 2004 and had shown good results. Further publications by de Menezes Ettinger and Alamo showed it to be an effective surgery in weight loss and in control of the metabolic syndrome in a South American cohort (7,8).
Methods
This is a prospective cohort study and was conducted between October 30th, 2014 and April 15th, 2016. Inclusion criteria was morbid obesity as defined as BMI ≥32 and/or those with type 2 diabetes mellitus as defined by ADA guidelines and associated with obesity (BMI >27) and uncontrolled with medical therapy were included. Excluded were patients who were <18 years of age and those with pregnancy, psychiatric illnesses and ongoing drug/alcohol abuse.
Patients were evaluated by a multi-disciplinary team including a dietician, psychologist, psychiatrist, physical trainer, physiotherapist and physicians.
Baseline measurements included body height, weight, BMI, body fat composition analysis, baseline blood studies, thyroid and liver function tests, lipid profile, a bone density measurement and an upper gastro-intestinal scopy.
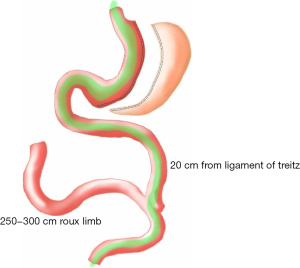
Surgical technique (Figure 1)
All patients were required to sign surgical consents before surgery. Surgery was performed under general anesthesia with the patients in a reversed Trendelenberg position. Four ports were used with an initial entry either with a Veress needle or with an optical bladeless trocar. A Fr 38 Oro-gastric tube was inserted and confirmed. The greater curvature, starting from 4 cm proximal to the pylorus, was devascularised with a 5 mm Ligasure (Covidien™) vessel sealing device. All attachments including the short gastric vessels were freed till the left crus was identified and displayed. Gastrectomy was carried out over a 38 Fr Oro-gastric tube with 60 mm Endo GIA (Covidien™) black cartridges for the first firing distally on the stomach followed by purple Tristapler reloads (Covidien™) proximally. The specimen of excised stomach was removed at the end of the procedure. The ligament of Trietz was identified and jejunum was divided at 20 cm with an Endo GIA stapler white cartridge (Covidien™). Distally, the jejunum was measured to a distance varying from 250 to 300 cm and was anastomosed to the (proximal) bilio-pancreatic jejunal limb with an Endo-GIA stapler white reload (Covidien™) and the enterotomy defect closed with 3-0 poliglecaprone continuous sutures. The mesentery defect was closed with 2-0 polyester sutures.
Post-operative care
Patient were started on oral liquids as sips on the day of the surgery and increased as tolerated. They were discharged when they could tolerate an adequate oral liquid diet. All were prescribed proton pump inhibitors for one month. Patients were followed up a week, a month, 3 months later and three monthly thereafter for the first year. Post-operatively, length of stay, major and minor complications were recorded. Weight, BMI and EWL, fasting sugar, HBA1C, C-peptide and clinical parameters were recorded a month, 3 month post op and every 3 months thereafter.
All data were recorded on and statistical analyses were performed using SPSS software version 15.0 (SPSS, Inc., Chicago, IL, USA). Characteristics were reported as mean and range for continuous variables and frequencies and percentages for categorical variables.
Results
Sixty five [65] patients under LPJB-SG during the duration of the study. Thirty three of them (50.76%) were females. The mean age was 38.72 (range, 19–71). The average BMI was 45.32 (range, 23.9–92.8). A total of 37 patients (56.9%) had T2DM. The mean duration of diabetes was 4.96 months (range, 1–15) and the mean HBA1C was 8.26 (range, 5.9–11.9). The average C-peptide level was 3.7 (range, 1.5–11.4). None of them were on Insulin injections (Table 1).
Table 1
| Characteristics | Number (N=65)/values | Range/% (SD) |
|---|---|---|
| Age (years) | 38.72 | 19–71 |
| Females | 33 | 50.7% |
| BMI preoperative | 45.3 | 27–92.8/11.74% |
| T2DM | 37 | 56.9% |
| Duration T2DM (months) | 4.96 | 1–15 |
| HBA1c | 8.26 | 5.9–11.9 |
| Fasting sugar (mg/dL) | 166.4 | 94–343/55.9% |
| C-peptide (ng/mL) | 3.7 | 1.5–11.4 (SD: 2.01) |
| Hypertension | 44 | 67.69% |
| Dyslipidaemia | 56 | 86.15% |
| Fatty liver | 63 | 96.92% |
Other co-morbidities were Hypertension in 44 (67.69%), hyperlipidaemia in 56 (86.15%) and fatty liver is 63 (96.92%). All surgeries were completed laparoscopically. The mean operating time was 93.13 minutes (41–180 minutes). The LOS was about 2.2 days on average (1–16 days).
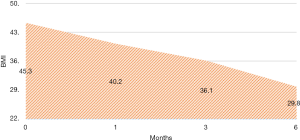
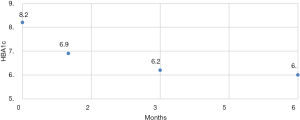
There were no intra-operative or immediate complications. The percentage of excess weight loss percentages (%EWL) were 26.44 (range, 4.7–167.4), 44.77 (19.7–169.3) and 65.87 (28.3–210.7) at 1, 3 and 6 months respectively (Figure 2). The mean HBA1C dropped from a mean of 6.9 preoperative to 6.04 at 6 months (Figure 3). For diabetic patients, at 1, 3 and 6 months, 11.53%, 60.86% and 66.66% patients respectively had achieved an HBA1C of <6.0 and stopped diabetic medications. The 13.33% had an HBA1C of <6.5 without OHA another 13.33% had an improvement in their glycaemic status while on OHA (Table 2). There were three complications. One patient presented with dehydration and another with a bleeding, the later surprisingly happened at 47 days post-op and both were managed conservatively with a brief hospitalization. There was one patient who presented with gastric stenosis and underwent a conversion to laparoscopic Roux-en-Y bypass successfully.
Table 2
| Variable | 1 month | 3 months | 6 months |
|---|---|---|---|
| HBA1C | |||
| Mean | 6.9 | 6.2 | 6.0 |
| Range | 5.3–9.2 | 5.2–9 | 4.8–8.2 |
| HBA1c <6.0, not on OHA | |||
| No. | 3 | 14 | 10 |
| Percentage | 11.5% | 60.8% | 66.6% |
| HBA1c <6.5, not on OHA | |||
| No. | 5 | 6 | 2 |
| Percentage | 19.2% | 26.0% | 13.3% |
| HBA1c <7.0, on OHA | |||
| No. | 5 | 1 | 2 |
| Percentage | 19.2% | 4.3% | 13.3% |
Discussion
Laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB) dominate the bariatric scene in the current era and have grown out of years of research and evidence. Both are effective in producing weight loss and in controlling co-morbidities. Laparoscopic gastric bypass is considered the gold standard with more than 80% of patients achieving greater than 70% EBW loss over 2 years (9). Remission of diabetes in impressive around 80% with a further 15% experiencing substantial improvement (10-13). LSG initially performed as a first stage procedure, has recently gained ground as an effective independent procedure. Its technical ease translates to a wider adoption worldwide and recent results show it to be as effective as gastric bypass (14,15) though some other studies have shown long term diabetes remission rates being worse (16).
LRYGP has a higher incidence of immediate and long term complications including anaemia, marginal ulcers, dumping syndrome and nutritional deficiencies that require lifelong supplements and surveillance. Other mal-absorptive procedures like bilio-pancreatic diversion, though gives the best results in terms of weight loss and remission of co-morbidities, is fraught with even higher nutritional problems, bowel disturbances and frequent hospital visits (17,18).
With the current knowledge of the roles played by intestinal hormones in obesity and diabetes, there is a much better and detailed understanding of the way modification of the gut anatomy produces weight loss and control of metabolic syndrome (6). In this context, it is apt to consider a newer procedure or a modification thereof, that can achieve similar results but which is devoid of long term complications, is less difficult to perform, and is reproducible and reversible.
Jejunal bypass performed along with sleeve gastrectomy was first described in 2004 and the concept was based on a restrictive procedure combined with a jejunal bypass of 300 cm with a Roux limb sutured to the remnant bypassed stomach. This was subsequently modified to excise the remnant stomach.
Alamo et al. had published their results in 2012 of 49 patients with type 2 DM and BMI <35 where they had reported a EWL of 76.1% at 18 months follow up. The remission of diabetes occurred in 81.6% of patients with a marked improvement in the remainder (19). There was no mortality and till the time followed up, no nutritional deficiencies were detected.
LPJB-SG subscribes to the prevalent and accepted theory of incretins in the remission of diabetes. By early exposure of the food to ileum, there is an increase in GLP-1 and Peptide YY secreted by the L cells of the ileum that has been postulated to decrease apoptosis of beta cells, enhance insulin secretion, decrease glucagon response and also decreases the gastric emptying time (20-23). These factors as well as others are responsible for control of diabetes seen in similar operations involving intestinal bypass. The easiest way to produce an early exposure of the food to the ileum is to bypass the intervening jejunum by anastomosing the very proximal jejunum to the ileum.
In the present study, a EWL of 65.87% and a significant improvement in glycaemic status in more than 90% of patients in 6 months shows this to be an effective procedure. Mean C-peptide levels of more than 3 ng/mL and hence good pancreatic function at the baseline would definitely have contributed to this excellent outcome. Sleeve gastrectomy in itself gives good results in terms of weight loss and control of metabolic syndrome. An addition of a mal-absorptive component such as a jejunal bypass would be additive in terms of sustenance of weight loss and definitely in the resolution and control of diabetes. This additive benefit, if it comes at a low cost in terms of operative time or the post-operative complication rate, would make this surgery a feasible and practicable option.
Given an intact pylorus and longer common channel and, it can be theorized that problems of dumping syndrome and malnutrition of macro/micro nutrients should occurs less. The creation of a blind loop of intestine might cause bacterial overgrowth and the blind loop syndrome. We had not seen any case in the follow-up presenting with symptoms of bacterial overgrowth. Alamo et al. consider this occurrence unlikely since there is no food transit through the bypassed bowel, which the resident bacteria can use as a substrate and that an iso-peristalitic orientation helps in mitigating against this possibility (19,24). The fact that the bowel anatomy can be easily reversed in the future if some complications happen due to the bypassed limb.
We believe that technical ease of the procedure, easy reproducibility, reversibility of bowel anatomy and comparable results would translate it to wider adoption worldwide soon. It’s a logical combination of excellent results from sleeve gastrectomy with minimal malabsorption to add in weight loss, would play an important role in remission of diabetes. Long term results and comparative studies with other surgeries in vogue are definitely required to validate this concept.
Conclusions
LPJB-SG is a feasible, safe and effective surgery for treatment of obesity and diabetes in this first Asia short-term result. It is technically simpler to perform and is easily reproducible. Long term and randomized controlled studies comparing with other surgical procedures would be required to assess it further.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.10.05). CKH serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jul 2016 to Jun 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and individual informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization (2013). Obesity and overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/
- Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol 2006;35:93-9. [Crossref] [PubMed]
- Haldar S, Chia SC, Henry CJ. Body Composition in Asians and Caucasians: Comparative Analyses and Influences on Cardiometabolic Outcomes. Adv Food Nutr Res 2015;75:97-154. [Crossref] [PubMed]
- Wang J, Thornton JC, Russell M, et al. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 1994;60:23-8. [PubMed]
- Lakdawala M, Bhasker A. Asian Consensus Meeting on Metabolic Surgery (ACMOMS). Report: Asian Consensus Meeting on Metabolic Surgery. Recommendations for the use of Bariatric and Gastrointestinal Metabolic Surgery for Treatment of Obesity and Type II Diabetes Mellitus in the Asian Population: August 9th and 10th, 2008, Trivandrum, India. Obes Surg 2010;20:929-36. [Crossref] [PubMed]
- Neff KJ, Olbers T, le Roux CW. Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med 2013;11:8. [Crossref] [PubMed]
- Alamo Alamo M, Sepúlveda Torres C, Zapata Perez L. Vertical isolated gastroplasty with gastro-enteral bypass: preliminary results. Obes Surg 2006;16:353-8. [Crossref] [PubMed]
- de Menezes Ettinger JE, Azaro E, Mello CA, et al. Analysis of the vertical isolated gastroplasty: a new bariatric operation. Obes Surg 2006;16:1261-3; author reply 1263-4. [Crossref] [PubMed]
- Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for Clinical Practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis 2008;4:S109-84. [Crossref] [PubMed]
- Goldfine AB, Patti ME. Diabetes improvement following Roux-en-Y gastric bypass: understanding dynamic changes in insulin secretion and action. Diabetes 2014;63:1454-6. [Crossref] [PubMed]
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248-256.e5. [Crossref] [PubMed]
- Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339-50; discussion 350-2. [Crossref] [PubMed]
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467-84; discussion 84-5. [PubMed]
- Biter LU, Gadiot RP, Grotenhuis BA, et al. The Sleeve Bypass Trial: a multicentre randomized controlled trial comparing the long term outcome of laparoscopic sleeve gastrectomy and gastric bypass for morbid obesity in terms of excess BMI loss percentage and quality of life. BMC Obes 2015;2:30. [Crossref] [PubMed]
- van Rutte PW, Luyer MD, de Hingh IH, et al. To Sleeve or NOT to Sleeve in Bariatric Surgery? ISRN Surg 2012;2012:674042.
- Rubino F, Moo TA, Rosen DJ, et al. Diabetes surgery: a new approach to an old disease. Diabetes Care 2009;32:S368-72. [Crossref] [PubMed]
- Søvik TT, Aasheim ET, Taha O, et al. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Ann Intern Med 2011;155:281-91. [Crossref] [PubMed]
- Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr 2009;90:15-22. [Crossref] [PubMed]
- Alamo M, Sepúlveda M, Gellona J, et al. Sleeve gastrectomy with jejunal bypass for the treatment of type 2 diabetes mellitus in patients with body mass index <35 kg/m2. A cohort study. Obes Surg 2012;22:1097-103. [Crossref] [PubMed]
- Ahrén B. Incretin dysfunction in type 2 diabetes: clinical impact and future perspectives. Diabetes Metab 2013;39:195-201. [Crossref] [PubMed]
- Dirksen C, Jørgensen NB, Bojsen-Møller KN, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 2012;55:1890-901. [Crossref] [PubMed]
- Rhee NA, Vilsbøll T, Knop FK. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes Obes Metab 2012;14:291-8. [Crossref] [PubMed]
- Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479-85. [Crossref] [PubMed]
- Bures J, Cyrany J, Kohoutova D, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol 2010;16:2978-90. [Crossref] [PubMed]
Cite this article as: Huang CK, Mahendra R, Hsin MC, Chang PC. Novel metabolic surgery: first Asia series and short-term results of laparoscopic proximal jejunal bypass with sleeve gastrectomy. Ann Laparosc Endosc Surg 2016;1:37.