
Transanal approach for rectal tumors: recent updates and future perspectives
Introduction
Local excision of rectal tumors has long been performed. The transsphincteric and transcoccygeal approaches had been used for local excision, especially for high-lying rectal tumors. The transcoccygeal (Kraske) approach requires mobilization of posterior pelvic floor muscles away from the coccyx to expose the rectum and the transsphincteric (York-Mason) approach involves complete division of the anal sphincter. With the development of new technologies for endoluminal operation, the transsphincteric and transcoccygeal approaches are rarely used today. Transanal excision (TAE) was first described by Parks as an alternative endoluminal treatment for certain rectal tumors in 1970 (1). After 10 years, anorectal surgical procedures with the use of different endoscopic devices into the anal canal were introduced. Transanal endoscopic microsurgery (TEM) was first described by Buess et al. in 1984 (2). A few years later, a newer and simpler alternative transanal endoscopic operation (TEO), was introduced and widely implemented. Maeda et al. (3,4) proposed a new transanal local excision procedure, minimally invasive transanal surgery (MITAS), for excising a proximal tumor at more distal sites. Recently, transanal minimally invasive surgery (TAMIS) has become increasingly more popular.
Selecting the appropriate surgical approach to rectal tumors is important. The approach must balance successful tumor eradication with functional implications for the patient. Owing to the significant morbidity and alterations in quality of life associated with rectal surgery (low anterior resection and abdominoperineal resection), a lot of time and research have been devoted to transanal approaches for rectal tumors. Several retrospective studies since the 1970s reported that TAE of early tumors with negative margins may provide similar outcomes those of radical resection (5). Since then, there had been many studies assessing the role of TAE of rectal cancer (6). Thereafter, the use of TAE had increased to 17.1% for T1 lesions and 11% for T2 lesions from 1989 to 2003 (7).
During the recent decades, total mesorectal excision (TME) has become the standard technique for the surgical treatment of rectal cancer (8). Nowadays, transanal TME (taTME) has been proposed as a new option in cases for which laparoscopic transabdominal TME (laTME) is difficult. TaTME is not a completely novel concept and it has benefited from previous experience of transabdominal-transanal (TATA) operations, TEM, TAMIS and natural orifice transluminal endoscopic surgery (NOTES) (9-11). Since the first taTME resection assisted with laparoscopy was reported in 2010 (12), taTME has shown promising results with regard to pathological quality and short- and mid-term outcomes (13-15).
Indication for TAE to rectal tumors
Local excision of rectal tumors has been advocated for premalignant lesions and used as definitive treatment for early rectal cancers in select groups without adverse prognostic features (16). Atypical rectal tumors such as neuroendocrine tumors and gastrointestinal stromal tumors are also usually approached through the transanal.
Local excision is suitable for Tis (carcinoma in situ) or T1 cancers with a favorable histology. The criteria for local treatment include T0 or Tis lesions; low-risk differentiated (well-to-moderately) T1 cancer; absence of lymphatic, vascular, or perineural invasion; and tumors ≤3 cm in diameter occupying ≤40% of the circumference of the rectal lumen. These principles apply to all local excision techniques. However, with the development of the technique and increased experience of surgeons, the indications have expanded. These technical approaches have extended beyond local excision, with the development of taTME being the most important in recent years. Additionally, for accurate patient selection, routine preoperative cardiopulmonary assessment, physical examination with digital rectal examination, fecal incontinence test, endoscopy, trans-rectal ultrasound (TRUS), and in case of malignancy; complete staging workup with pelvic magnetic resonance imaging and abdominal computed tomography, are recommended. For determining local excision, local T staging should be accurate. TRUS allows predicting early T1 lesions that might be suitable for local excision. Hildebrandt et al. (17) proposed a preoperative tumor staging system based on ultrasonic determination of the infiltrative depth of tumors, so-called uTNM, and this technique contribute to a more accurate determination of the depth of invasion with classification of the rectal wall layer.
Techniques for the transanal approach
- Conventional TAE.
- MITAS.
- Transanal endoscopic surgery (TES).
- TEM;
- TEO;
- TAMIS;
- taTME.
TAE
TAE has been the mainstay of treatment for many years. TAE is a simple method that can be easily performed if the tumor is located in the anal canal and easily accessible under adequate exposure of the anal canal. There is no need for additional equipment, and it can be performed in the outpatient department. An additional benefit to this approach is the minimal, if any, compromise of anorectal and urogenital function. These factors make TAE the most common method of local excision (18).
Conventional TAE is often limited to tumors ≤4 cm in diameter that lie within 6–8 cm of the anal verge (16). Lesions in the middle and upper rectum are usually inaccessible with TAE because of their distance from the anal verge, and attempted excisions are hampered by inadequate surgical exposure, confinement of the operating field, and uncertainty of a clear surgical resection margin (19).
Patients should undergo preoperative assessment including digital rectal examination and rigid sigmoidoscopy to confirm location and mobility. Patients receive a cleansing enema the day before the operation; prophylaxis with antibiotics and antithrombotics is usually recommended. After the induction of local, regional, or general anesthesia, the patient is placed in position. Some authors additionally propose pudendal nerve block for sphincter relaxation. The positioning of the patient is dependent on the preference of the surgeon; however, the orientation of the lesion is usually the deciding factor, with preference taken to operating downward. Most operations are performed with the patient in the prone jackknife position; however, some posteriorly located lesions may be better approached with the lithotomy position. The perianal area is exposed by taping the buttocks apart; the lesion is exposed with direct vision by using a Hill Ferguson, Park, or Barr retractor. Traction sutures can be placed distal to the lesion to improve visualization. The calculated excision margin (10 mm) is typically marked by using electrocautery in a circumferential pattern around the lesion. The specimen must be carefully taken so as not to manipulate the lesion or handle it with instruments. After a full thickness excision of the lesion, the specimen is oriented on a needle board and sent to the pathology laboratory. After irrigation, the defect can be either left open or closed transversely with absorbable sutures. If the lesion is posterior to the rectum and above the puborectalis muscle, the defect is closed with absorbable sutures. In case of anteriorly located lesions, repair should be conducted to avoid injury of adjacent structures, such as the prostate, urethra, or vagina. At the end of the procedure, a proctoscopic examination is essential to confirm that the rectal lumen was not inadvertently closed or narrowed.
Complications associated with TAE include urinary retention, urinary tract infection, infections of the perirectal and ischiorectal space, fecal impactions, and delayed hemorrhage. Nevertheless, the incidences of these complications and mortality are very low. TAE has several limitations. In general, this approach is technically difficult with higher lesions owing to poor visualization. More important, visualization during TAE can be suboptimal, which can affect the quality of an oncologic resection margin. Concerns have also been raised about the high rates of tumor fragmentation and recurrence with TAE. The high rates of recurrence are likely due to rates of margin positivity that exceed 10% in even the most experienced and expert case series (6,18). Patients treated with TAE compared with those treated with radical surgery for T1 rectal cancer had a significantly higher 5-year local recurrence rate (12% vs. 6%) and lower 5-year survival rate (70% vs. 80%) and 5-year disease-free rate (64% vs. 77%) (20).
MITAS
A new transanal local excision procedure, MITAS, has been developed with a specially designed anal retractor connected to the Octopus retractor holder, a stapler device, and several newly developed techniques to excise a proximal tumor at a more distal site (21).
Operation was usually performed under spinal anesthesia with the patient in lithotomy or jackknife position, according to the site of tumor (4). The procedure consists of inserting into the rectum an originally designed E- or F-type anal retractor [a modified K-type anal retractor (22); Yufu Itonaga Co Ltd.] connected to the Octopus retractor holder, long type, 22 inches (Mednosbro AG).
To enable excision of a proximal tumor at a more accessible site, shortening or roll-in technique, intussusception, or invagination technique with retraction stitches is used. The retractor is inserted into the anus and rolled to pull the rectum in and permit easy access to the tumor in the proximal rectum (3). When the tumor is still beyond the surgical field, the retractor is opened and fixed and two Babcock forceps are used to pull the tumor gradually (21). Retraction stitches are passed under the tumor, from one side to the other with a minimum macroscopic margin of 5 mm from the boundary of the tumor to the adjacent normal mucosa by a 36-mm-long atraumatic needle with absorbable 1-0 thread, enough to retract the tumor fully and pull the rectum down. ENDO GIA (Tyco Co Ltd.) is used for excision and anastomosis while fully retracting the rectum with retraction stitches distally. Application of a stapler is usually done transversely, but oblique application is sometimes needed because of the difficult angle in the rectum or size of the tumor.
TES
TEM
Gerhard Buess of Germany pioneered TEM (Richard Wolf, Germany) in the early 1980s as a minimally invasive technique allowing the resection of adenomas and early rectal carcinomas unsuitable for local or colonoscopy excision, which would otherwise require major surgery (23). This method was basically designed from the idea of laparoscopic surgical techniques so-called because it is a minimally invasive technique and has proved to be useful for treating lesions in the mid or upper rectum. The main indications for TEM are rectal tumors that are out of reach for TAE and are unsuitable for endoscopic removal (24). In addition, this technique can even be extended to lesions in the low sigmoid colon, with success reported up to 20 cm from the anal verge (25). Since its development, TEM has been also used for a variety of other rectal pathologies including neuroendocrine tumors, rectal prolapse, early stage carcinomas, and palliative resection of rectal cancers (26).
The orientation of the lesion is usually the deciding factor for the positioning. The lesion needs to be situated at the 180 degree angle of the scope view. For a posterior lesion, the patient is placed in a lithotomy position. For an anterior lesion, the patient is placed in the prone jackknife position. The operative technique for TEM involves three main components: a rigid operating rectoscope, a laparoscopic camera, and modified laparoscopic instruments. The operating rectoscope is typically 4 cm in diameter and varies from 12 to 20 cm in length. The rectoscope maintains an airtight seal at the anus once inserted in the rectum, and is held in place by the obligate articulating arm, which fixes the rectoscope to the operating table. The rectoscope has a port for the inflow of CO2 for the pneumorectum, and an outflow for smoke evacuation during cauterization. The faceplate on the rectoscope has four ports through which a stereotactic telescope with connection to a three-dimensional video system and three modified laparoscopic instruments are connected to facilitate dissection and suturing (26). While the instruments are specialized, the operative steps are otherwise no different than for TAE. The lesion is then centered in the scope view. By using scissors, cautery, and graspers, a dotted line is burned around the target lesion with a 10-mm margin. Care must be taken to avoid trauma to and fragmentation of the specimen. After homeostasis is obtained, the defect is closed with absorbable full-thickness sutures in a transverse line. The defect can be left open to heal secondarily if the defect is posterior and surrounded by mesorectal fat. An anterior defect must be closed; closure is facilitated by placing a central stay suture and sewing from each corner to avoid tension on the suture line.
The limitation of TEM is that the equipment is designed to operate from the top-down; thus, the lesion must be oriented toward the floor to be compatible with the equipment. This means that the patient’s positioning is dependent on the tumor location, and sometimes specialized split-leg operating tables are necessary to resect anterior tumors requiring the prone jackknife position. Distal lesions near the sphincter are difficult to excise with TEM owing to the configuration of the equipment and inability to maintain insufflations of CO2 to distend the rectum. This is the reason why TAE is easier to use for low-lying lesions. Other limitations to TEM focus on specialized equipment, which have a steep learning curve and high associated costs for the hospital (27,28).
TEO
A few years later a cheaper alternative was introduced compared with TEM. The TEO (Karl Storz, Germany), which was a newer and simpler system, has become widely implemented. Indication of TEO is similar to TEM.
TEO platform was performed in a lithotomy position using a 30° forward-oblique telescope, adequate adjustment of the rectoscope, and curved laparoscopic operating instruments. TEO procedure was carried out under general anesthesia. After installation of the holding system, the anus was gently dilated, and the operating rectoscope (7.5 or 15 cm long, 4 cm in diameter) with obturator was inserted in the rectum with copious lubricant and fastened to the support arm attached to the operating table. The working attachment used with the rectoscope had two channels for instruments of 5 mm and one channel for instruments up to 12 mm. After achieving the pneumorectum with insufflations of CO2 to 12 mmHg or more, a high definition (HD) 5-mm diameter endoscope with fiber-optic light transmission and 30° angled view was inserted through a 5-mm endoscope channel and the rectoscope was adjusted to achieve the best position for procedures (Figure 1A). Except the instruments are specialized, the operative steps are otherwise no different than those for TEM.
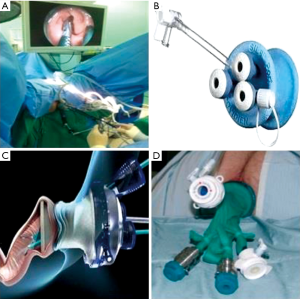
Similar to TEM, lesions near the anal verge are difficult to excise with the TEO. Standard laparoscopic instruments, equipment, and set up costs are lower, potentially opening the technique to any surgeon with previous laparoscopic experience. Hur et al. (29) performed initial experience of TEO and reported the mean operative time was 85 minutes, and the mean postoperative hospital stay was 4.5 days, a positive resection margin was documented for 9% patients. Furthermore, they demonstrated that according to the cumulative sum analysis, the operation time and hospital stay significantly decreased after 17 case experiences (29). Several studies have compared TEO with TEM for benign and malignant lesions and have shown satisfactory outcomes (30,31).
TAMIS
In recent years, TAMIS has become increasingly more popular. Reported by Atallah et al. (32) in 2010, the technique stems from the use of a single port initially designed for abdominal surgery. Since the inception of TAMIS, at least 390 procedures were reported worldwide from 2010 to 2013 (33). This technique uses a single, disposable, multichannel port inserted into the anus as opposed to the rigid operating rectoscope. Currently in the United States, two ports are approved for TAMIS by the Food and Drug Administration: single-incision assisted laparoscopic surgery port (Covidien, USA; Figure 1B) and GelPOINT Path Transanal Access Platform (Applied Medical, USA; Figure 1C).
The indications of TAMIS are the same as those of other TES. The main benefits of TAMIS are the relative ease of use and low cost, owing to the use of conventional laparoscopic instruments, including a laparoscopic camera, graspers, energy sources, and a standard laparoscopic CO2 insufflator. It is ideal for lesions at 8–12 cm from the anal verge; however, it has been successfully performed in the lower and mid rectum (34). Distal lesions are covered by the transanal port, and excision of very proximal lesions can cause entry into the peritoneal cavity.
Mechanical bowel preparation or enema, antibiotics and antithrombotic prophylaxis are usually recommended. Anesthesia may be general or spinal. Lee et al. (35) have reported a series of 25 TAMIS procedures in which spinal anesthesia was used. In TAMIS, most lesions can be excised with a lithotomy position. However, we still recommend the prone position for the patients with large anterior lesions, especially if the distance from the anal verge is in a range in which there might be a risk of perforating the peritoneum. After the patient is positioned and the port is placed, pneumorectum is achieved with the standard CO2 insufflator, with pressures ranging from 15 to 25 mmHg. Several different cameras can be employed, including those with a flexible tip. One common practice is to use the 5-mm, 30-degree bariatric camera with a right-angle light cord adaptor. Conventional graspers, scissors, and electrocautery devices are employed, along with ultrasonic or bipolar energy devices as needed. Owing to the technical challenges of suturing in this confined working space, many techniques similar to TEM have been employed, including the use of clips and beads, barbed suture, and specialized suturing devices.
When compared with other platforms, TAMIS has several advantages. Devices that are used for TAMIS are more pliable than the 40-mm rigid scope used for TEM, and possibly lead to less impairment of sphincter function; the set-up time is significantly lower for TAMIS. Use of regular conventional laparoscopic instruments, as opposed to the fixed eyepiece of the TEM rectoscope, enables advancing the scope into the proximal rectum to look beyond the tumor. TAMIS is easily learned by surgeons because of its simplicity and similarity with conventional laparoscopic surgery, and it is a cost effective alternative to TEM (32). For some authors, the introduction of the TAMIS port into the anal canal makes it more complex than TEM or TEO (36). A disadvantage of TAMIS is that the rectoscope cannot be mobilized at the site of the lesion; rectal lesions behind a rectal haustral valve may be more difficult to access and remove. The longer channels associated with TEM and TEO equipment facilitates intraluminal rectal retraction. Moreover, an assistant is required to hold and manipulate the laparoscope during the TAMIS procedure.
The authors who introduced TAMIS went on to describe the use of a robotic platform for TAMIS in a cadaveric model in 2011 (37), and then extended that robotic TAMIS platform to live patients in 2012 (38). Since then, its use in in humans has been described with both the GelPOINT Path platform (Applied Medical, USA) and a glove port (Figure 1D) (39).
The authors suggested that the transanal glove port facilitated the robotic setup, enabling flexibility and allowing docking of the cannulas away from the limited perianal workspace (40). Furthermore, the glove port provided a wider axis of movement for instruments inside the rectum, or allowed them to be easily rotated and/or crossed. Although robotic TAMIS has been shown to be feasible, this technique is still relatively new, and more studies are necessary before to widespread adoption.
Postoperative complication of TES
Regardless of procedures, the morbidity and mortality are lower than for radical surgery. Operative mortality is less than 0.5% and morbidity ranges from 4% to 30% in large series, depending on the inclusion of minor complications. The most frequent complications include acute urinary retention, bleeding requiring reoperation, abdominal perforation and recto-vaginal fistula (41). Kumar et al. (42) found that complications correlate with tumors located laterally and more than 8 cm from the anal verge. Pelvic sepsis, which occurs in about 3% of cases, is more common in lesions within 2 cm of the dentate line. The conversion rate to TAE is around 5%, and the main reason for conversion is technical difficulties (24,26). Peritoneal perforation, which was thought to represent a complication requiring conversion to laparotomy, can usually be salvaged with TES for experienced surgeons (43,44). A multicenter study by Baatrup et al. (45), performed by using database of 888 TEM procedures, found 22 perforations in the peritoneal cavity. They reported no association with major short term complications or adverse long-term oncological outcomes (45).
Outcomes of TES
Radical surgery with TME is still the treatment of choice for rectal cancer, offering patients the best results in terms of local recurrence and survival (46). However, TME is associated with significant mortality and morbidity (47). According to the experience in the last decades, TEM and TEO have been accepted as effective treatments in selected patients with early rectal cancer, with similar oncologic outcomes to and better functional effects than those of radical surgery (48,49). Recently, TAMIS has been proposed as an alternative technique; however, the experience with this approach for rectal cancer is still limited because of short follow-up (27,33,50-55).
TEM has a lower positive resection margin rate than TAE, with less fragmented specimens and better oncologic outcomes (56,57). Elmessiry et al. (50) showed that TAE was an independent predictor of local recurrence compared with TEM. The rate of reported positive resection margin in the surgical specimen in TAMIS was 4.4–6% (27,33,54), similar to those obtained with TEM, and seems to be related with the T stage (26,58,59). Some studies have compared TEM with radical surgery in early rectal cancer, showing similar results in terms of local recurrence and survival (60,61). In a meta-analysis, Winde et al. (62) demonstrated that the rate of local recurrence was higher with TEM (12% vs. 0.5%); however, no difference in survival was found.
TES seems to be a reasonable alternative to radical surgery in patients with low-risk rectal cancer (26,41,47,48,52,61,63,64) with local recurrence rates ranging from 0% to 39%. These wide differences in range can be explained by the heterogeneity of cases, different selection criteria, risk characteristics, and surgical techniques; however, most local recurrence rates are under 10% (24,26,60,62,65-70). TES alone is not appropriate for patients with T2 or worse tumors, considering that the risk of local recurrence varies between 9.5% and 47% (24,26,47,52,58-61,64,68-72). However, even in these studies there are considerable differences between low- and high-risk cancers (73). TES may be performed on patients with high-risk T1 or T2–3 tumors with poor life expectancy and severe morbidity, or those unfit for major surgery, or simply as palliative treatment in case of disseminated disease (47,61,63).
Salvage surgery for recurrence after TES demonstrated disappointing oncologic outcomes; the stage is usually more advanced than in primary lesions and may require multivisceral resection and an ostomy in up to 43% of cases. Survival is seriously compromised, with a 5-year survival ranging from 43% to 68%, dropping to 29% in patients with unfavorable histology (63,68,74). In contrast, Levic et al. did not find any difference in outcome between patients with rectal cancer undergoing immediate salvage TME after TEM and those undergoing primary TME (75). Despite contrasting conclusions, all authors warned that perforations into the original operating field during subsequent TME can occur owing to fibrotic changes to the bowel wall, which might allow microscopic tumor spillage.
Future perspectives
Several new techniques and approaches are still under investigation, currently in preclinical or experimental stages, such as transanal natural orifice transluminal endoscopic surgery (NOTES), taTME, and robotic-TES (26,51,76). TES platforms seem to be safe for both transanal NOTES and taTME (77,78). Robotic technology can lower the difficulty inherent in TES platforms (79). However, clinical trials are necessary for full evaluation of these techniques.
taTME
The latest development in transanal approaches is taTME. Transanal TME was introduced in 2010 with the aim to cope with all these limits and improve the quality of mesorectal dissection even in the most challenging cases (11). Dissection of the distal rectum according to TME principles may be somewhat cumbersome in cases of narrow pelvis, bulging tumors, and obese patients. In adequate exposure and loss of the good plane of dissection require finding another alternative treatment approach. During the last decade, transanal approaches have been extensively used to overcome the inherent shortcomings of laTME (69,77). Among these emerging transanal techniques, taTME is a new minimally invasive procedure with the essential aim of improving oncological treatment quality and avoiding pelvic nerve injury in patients with mid or low rectal cancer. Given the encouraging outcomes of systematic investigation of taTME for patients with rectal cancer (80,81), taTME may be optimized as a surgical approach for rectal cancer. In comparison with conventional laTME, taTME defines the distal resection margin more precisely, with better visualization of the distal rectum, and allows the surgeon to perform deep pelvic dissection without the need for difficult retraction (82). However, the benefits of taTME compared with laTME must be confirmed before conducting multicenter randomized controlled trials (RCTs) and unifying taTME procedures. According to a meta-analysis study, the percentage of patients with complete mesorectum was 83.4% in the taTME group and 73.4% in the laTME group (83). In addition, achievement of complete plus near complete mesorectum was also greater in the taTME group (95.3% vs. 88.2%) (83). Hence, for patients with mid or low rectal cancer, taTME may achieve a complete or near complete resection of the mesorectum relative easily, compared with laTME. However, whether a higher quality of mesorectal resection will result in good survival remains unknown. Safety is always the most important issue for a new technique. The meta-analysis indicated a comparable rate of intraoperative complications and a significantly lower incidence of postoperative complications in the taTME group than in the laTME group (83).
As the new surgical technique of taTME is adopted increasingly by surgeons, the patient selection criteria will be crucial and will continue to inspire debate. Of note, the protocol published recently for a multicenter RCT comparing taTME with laTME (COLOR III) has formulated strict criteria for patient selection (84). According to the selection criteria of this protocol, T3 tumors with margins <1 mm to the endopelvic fascia, tumors with ingrowth in the internal sphincter or levator ani muscle, and all T4 tumors staged before preoperative therapy were excluded (84). However, the nature of the surgical candidates best suited to taTME treatment requires further studies.
In a matched case-control study from Taiwan, Chen et al. (85) demonstrated that compared with laTME, taTME not only achieves identical circumferential margin status without compromising other operative and quality parameters but also benefits patients by achieving a longer distal margin. Additionally, Denost et al. (86) performed a randomized trial in France, and reported that the rate of positive circumferential resection margin decreased significantly after taTME compared with abdominal low rectal dissection (4% vs. 18%; P=0.025). Currently, RCTs examining taTME are under way; the COLOR III study has been designed to compare taTME versus laTME for mid and low rectal cancer. TaTME is expected to be superior to laTME in terms of clear circumferential resection margins in case of mid and low rectal carcinomas (84). Although taTME is another new technique with great promise, the supporting data are preliminary, and further studies with larger cohorts of patients are needed to evaluate long term functional and oncological outcomes.
Conclusions
There have been significant advances in the transanal approach in the last 30 years. Appropriate patient selection is the key for good outcomes. These techniques have enabled mid and upper rectal lesion and sphincter salvage, leading to a better quality of life. From the point of view of technical advancement, it would be better to adopt this technique as a treatment option, and prospectively randomized comparison clinical trials should be conducted. When we are planning treatment for patients with early rectal cancer, the risk of local recurrence must be balanced with the quality of life. Nowadays, transanal approaches including taTME should be considered as good options for the treatment of rectal cancer because these techniques are definitely useful in selected patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.11.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parks AG, Stuart AE. The management of villous tumours of the large bowel. Br J Surg 1973;60:688-95. [Crossref] [PubMed]
- Buess G, Hutterer F, Theiss J, et al. A system for a transanal endoscopic rectum operation. Chirurg 1984;55:677-80. [PubMed]
- Maeda K, Hashimoto M, Nakajima K, et al. Transanal surgery with a new anal retractor and a stapler for tumours in the proximal rectum. Eur J Surg 1997;163:219-21. [PubMed]
- Maeda K, Maruta M, Utsumi T, et al. Minimally invasive transanal surgery for localized rectal carcinoid tumors. Tech Coloproctol 2002;6:33-6. [Crossref] [PubMed]
- Morson BC, Bussey HJ, Samoorian S. Policy of local excision for early cancer of the colorectum. Gut 1977;18:1045-50. [Crossref] [PubMed]
- Paty PB, Nash GM, Baron P, et al. Long-term results of local excision for rectal cancer. Ann Surg 2002;236:522-29; discussion 529-30. [Crossref] [PubMed]
- You YN, Baxter NN, Stewart A, et al. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg 2007;245:726-33. [Crossref] [PubMed]
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Marks JH, Frenkel JL, D'Andrea AP, et al. Maximizing rectal cancer results: TEM and TATA techniques to expand sphincter preservation. Surg Oncol Clin N Am 2011;20:501-20. viii-ix. [Crossref] [PubMed]
- Atallah SB, Larach S, deBeche-Adams TC, et al. Transanal minimally invasive surgery (TAMIS): a technique that can be used for retrograde proctectomy. Dis Colon Rectum 2013;56:931. [Crossref] [PubMed]
- de Lacy AM, Rattner DW, Adelsdorfer C, et al. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: "down-to-up" total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc 2013;27:3165-72. [Crossref] [PubMed]
- Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 2010;24:1205-10. [Crossref] [PubMed]
- Lacy AM, Tasende MM, Delgado S, et al. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J Am Coll Surg 2015;221:415-23. [Crossref] [PubMed]
- Veltcamp Helbach M, Deijen CL, Velthuis S, et al. Transanal total mesorectal excision for rectal carcinoma: short-term outcomes and experience after 80 cases. Surg Endosc 2016;30:464-70. [Crossref] [PubMed]
- Muratore A, Mellano A, Marsanic P, et al. Transanal total mesorectal excision (taTME) for cancer located in the lower rectum: short- and mid-term results. Eur J Surg Oncol 2015;41:478-83. [Crossref] [PubMed]
- Beck DE, Roberts PL, Saclarides TJ, et al. editors. The ASCRS textbook of colon and rectal surgery. Springer Science & Business Media, 2011.
- Hildebrandt U, Feifel G. Preoperative staging of rectal cancer by intrarectal ultrasound. Dis Colon Rectum 1985;28:42-6. [Crossref] [PubMed]
- Garcia-Aguilar J, Mellgren A, Sirivongs P, et al. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg 2000;231:345-51. [Crossref] [PubMed]
- Neary P, Makin GB, White TJ, et al. Transanal endoscopic microsurgery: a viable operative alternative in selected patients with rectal lesions. Ann Surg Oncol 2003;10:1106-11. [Crossref] [PubMed]
- Endreseth BH, Myrvold HE, Romundstad P, et al. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum 2005;48:1380-8. [Crossref] [PubMed]
- Maeda K, Maruta M, Sato H, et al. Outcomes of novel transanal operation for selected tumors in the rectum. J Am Coll Surg 2004;199:353-60. [Crossref] [PubMed]
- Maeda K, Hashimoto M, Katai H, et al. Peranal introduction of the stapler in colorectal anastomosis with a double-stapling technique. Br J Surg 1994;81:1057. [Crossref] [PubMed]
- Buess G, Theiss R, Hutterer F, et al. Transanal endoscopic surgery of the rectum - testing a new method in animal experiments. Leber Magen Darm 1983;13:73-7. [PubMed]
- Dias AR, Nahas CS, Marques CF, et al. Transanal endoscopic microsurgery: indications, results and controversies. Tech Coloproctol 2009;13:105-11. [Crossref] [PubMed]
- Gordon PH, Nivatvongs S. editors. Principles and practice of surgery for the colon, rectum, and anus. CRC Press, 2007.
- Heidary B, Phang TP, Raval MJ, et al. Transanal endoscopic microsurgery: a review. Can J Surg 2014;57:127-38. [Crossref] [PubMed]
- McLemore EC, Weston LA, Coker AM, et al. Transanal minimally invasive surgery for benign and malignant rectal neoplasia. Am J Surg 2014;208:372-81. [Crossref] [PubMed]
- Saclarides TJ. editor. The history of transanal endoscopic surgery. Seminars in Colon and Rectal Surgery. Elsevier, 2015.
- Hur H, Bae SU, Han YD, et al. Transanal Endoscopic Operation for Rectal Tumor: Short-term Outcomes and Learning Curve Analysis. Surg Laparosc Endosc Percutan Tech 2016;26:236-43. [Crossref] [PubMed]
- Nieuwenhuis DH, Draaisma WA, Verberne GH, et al. Transanal endoscopic operation for rectal lesions using two-dimensional visualization and standard endoscopic instruments: a prospective cohort study and comparison with the literature. Surg Endosc 2009;23:80-6. [Crossref] [PubMed]
- Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, et al. Transanal endoscopic microsurgery with 3-D (TEM) or high-definition 2-D transanal endoscopic operation (TEO) for rectal tumors. A prospective, randomized clinical trial. Int J Colorectal Dis 2014;29:605-10. [Crossref] [PubMed]
- Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 2010;24:2200-5. [Crossref] [PubMed]
- Martin-Perez B, Andrade-Ribeiro GD, Hunter L, et al. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol 2014;18:775-88. [Crossref] [PubMed]
- deBeche-Adams T, Nassif G. Transanal Minimally Invasive Surgery. Clin Colon Rectal Surg 2015;28:176-80. [Crossref] [PubMed]
- Lee TG, Lee SJ. Transanal single-port microsurgery for rectal tumors: minimal invasive surgery under spinal anesthesia. Surg Endosc 2014;28:271-80. [Crossref] [PubMed]
- Barendse RM, Doornebosch PG, Bemelman WA, et al. Transanal employment of single access ports is feasible for rectal surgery. Ann Surg 2012;256:1030-3. [Crossref] [PubMed]
- Atallah SB, Albert MR, deBeche-Adams TH, et al. Robotic TransAnal Minimally Invasive Surgery in a cadaveric model. Tech Coloproctol 2011;15:461-4. [Crossref] [PubMed]
- Atallah S, Parra-Davila E, DeBeche-Adams T, et al. Excision of a rectal neoplasm using robotic transanal surgery (RTS): a description of the technique. Tech Coloproctol 2012;16:389-92. [Crossref] [PubMed]
- Hompes R, Rauh SM, Ris F, et al. Robotic transanal minimally invasive surgery for local excision of rectal neoplasms. Br J Surg 2014;101:578-81. [Crossref] [PubMed]
- Hompes R, Ris F, Cunningham C, et al. Transanal glove port is a safe and cost-effective alternative for transanal endoscopic microsurgery. Br J Surg 2012;99:1429-35. [Crossref] [PubMed]
- Lartigau C, Lebreton G, Alves A. Local resection for small rectal cancer. J Visc Surg 2013;150:325-31. [Crossref] [PubMed]
- Kumar AS, Coralic J, Kelleher DC, et al. Complications of transanal endoscopic microsurgery are rare and minor: a single institution's analysis and comparison to existing data. Dis Colon Rectum 2013;56:295-300. [Crossref] [PubMed]
- Marks JH, Frenkel JL, Greenleaf CE, et al. Transanal endoscopic microsurgery with entrance into the peritoneal cavity: is it safe? Dis Colon Rectum 2014;57:1176-82. [Crossref] [PubMed]
- Morino M, Allaix ME, Famiglietti F, et al. Does peritoneal perforation affect short- and long-term outcomes after transanal endoscopic microsurgery? Surg Endosc 2013;27:181-8. [Crossref] [PubMed]
- Baatrup G, Borschitz T, Cunningham C, et al. Perforation into the peritoneal cavity during transanal endoscopic microsurgery for rectal cancer is not associated with major complications or oncological compromise. Surg Endosc 2009;23:2680-3. [Crossref] [PubMed]
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. [Crossref] [PubMed]
- Lezoche G, Paganini AM, Campagnacci R, et al. Treatment of rectal cancer by transanal endoscopic microsurgery: review of the literature. Minerva Chir 2013;68:1-9. [PubMed]
- Monson JR, Weiser MR, Buie WD, et al. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum 2013;56:535-50. [Crossref] [PubMed]
- Morino M, Risio M, Bach S, et al. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc 2015;29:755-73. [Crossref] [PubMed]
- Elmessiry MM, Van Koughnett JA, Maya A, et al. Local excision of T1 and T2 rectal cancer: proceed with caution. Colorectal Dis 2014;16:703-9. [Crossref] [PubMed]
- Smart CJ, Cunningham C, Bach SP. Transanal endoscopic microsurgery. Best Pract Res Clin Gastroenterol 2014;28:143-57. [Crossref] [PubMed]
- Kidane B, Chadi SA, Kanters S, et al. Local resection compared with radical resection in the treatment of T1N0M0 rectal adenocarcinoma: a systematic review and meta-analysis. Dis Colon Rectum 2015;58:122-40. [Crossref] [PubMed]
- Morino M, Arezzo A, Allaix ME. Transanal endoscopic microsurgery. Tech Coloproctol 2013;17:S55-61. [Crossref] [PubMed]
- Albert MR, Atallah SB, deBeche-Adams TC, et al. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum 2013;56:301-7. [Crossref] [PubMed]
- Lim SB, Seo SI, Lee JL, et al. Feasibility of transanal minimally invasive surgery for mid-rectal lesions. Surg Endosc 2012;26:3127-32. [Crossref] [PubMed]
- Moore JS, Cataldo PA, Osler T, et al. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum 2008;51:1026-30; discussion 1030-1. [Crossref] [PubMed]
- Clancy C, Burke JP, Albert MR, et al. Transanal endoscopic microsurgery versus standard transanal excision for the removal of rectal neoplasms: a systematic review and meta-analysis. Dis Colon Rectum 2015;58:254-61. [Crossref] [PubMed]
- Morino M, Allaix ME. Transanal endoscopic microsurgery: what indications in 2013? Gastroenterol Rep (Oxf) 2013;1:75-84. [Crossref] [PubMed]
- Allaix ME, Arezzo A, Giraudo G, et al. Transanal endoscopic microsurgery vs. laparoscopic total mesorectal excision for T2N0 rectal cancer. J Gastrointest Surg 2012;16:2280-7. [Crossref] [PubMed]
- Hershman MJ, Mohammad H, Hussain A, et al. Local excision of rectal tumours by minimally invasive transanal surgery. Br J Hosp Med (Lond) 2013;74:387-90. [Crossref] [PubMed]
- Sajid MS, Farag S, Leung P, et al. Systematic review and meta-analysis of published trials comparing the effectiveness of transanal endoscopic microsurgery and radical resection in the management of early rectal cancer. Colorectal Dis 2014;16:2-14. [Crossref] [PubMed]
- Winde G, Nottberg H, Keller R, et al. Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum 1996;39:969-76. [Crossref] [PubMed]
- Heafner TA, Glasgow SC. A critical review of the role of local excision in the treatment of early (T1 and T2) rectal tumors. J Gastrointest Oncol 2014;5:345-52. [PubMed]
- Damin DC, Lazzaron AR. Evolving treatment strategies for colorectal cancer: a critical review of current therapeutic options. World J Gastroenterol 2014;20:877-87. [Crossref] [PubMed]
- Sgourakis G, Lanitis S, Gockel I, et al. Transanal endoscopic microsurgery for T1 and T2 rectal cancers: a meta-analysis and meta-regression analysis of outcomes. Am Surg 2011;77:761-72. [PubMed]
- Morino M, Allaix ME, Caldart M, et al. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc 2011;25:3683-90. [Crossref] [PubMed]
- Guerrieri M, Gesuita R, Ghiselli R, et al. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J Gastroenterol 2014;20:9556-63. [PubMed]
- Doornebosch PG, Ferenschild FT, de Wilt JH, et al. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum 2010;53:1234-9. [Crossref] [PubMed]
- Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, et al. Transanal endoscopic surgery in rectal cancer. World J Gastroenterol 2014;20:11538-45. [Crossref] [PubMed]
- Borschitz T, Heintz A, Junginger T. The influence of histopathologic criteria on the long-term prognosis of locally excised pT1 rectal carcinomas: results of local excision (transanal endoscopic microsurgery) and immediate reoperation. Dis Colon Rectum 2006;49:1492-506; discussion 1500-5. [Crossref] [PubMed]
- Stipa F, Burza A, Lucandri G, et al. Outcomes for early rectal cancer managed with transanal endoscopic microsurgery: a 5-year follow-up study. Surg Endosc 2006;20:541-5. [Crossref] [PubMed]
- Bhangu A, Brown G, Nicholls RJ, et al. Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann Surg 2013;258:563-9; discussion 569-71. [PubMed]
- Borschitz T, Kneist W, Gockel I, et al. Local excision for more advanced rectal tumors. Acta Oncol 2008;47:1140-7. [Crossref] [PubMed]
- Bikhchandani J, Ong GK, Dozois EJ, et al. Outcomes of salvage surgery for cure in patients with locally recurrent disease after local excision of rectal cancer. Dis Colon Rectum 2015;58:283-7. [Crossref] [PubMed]
- Levic K, Bulut O, Hesselfeldt P, et al. The outcome of rectal cancer after early salvage TME following TEM compared with primary TME: a case-matched study. Tech Coloproctol 2013;17:397-403. [Crossref] [PubMed]
- Aly EH. SILS TEM: The new armamentarium in transanal endoscopic surgery. J Minim Access Surg 2014;10:102-3. [PubMed]
- Wolthuis AM, de Buck van Overstraeten A, D'Hoore A. Laparoscopic natural orifice specimen extraction-colectomy: a systematic review. World J Gastroenterol 2014;20:12981-92. [Crossref] [PubMed]
- Araujo SE, Crawshaw B, Mendes CR, et al. Transanal total mesorectal excision: a systematic review of the experimental and clinical evidence. Tech Coloproctol 2015;19:69-82. [Crossref] [PubMed]
- Gómez Ruiz M, Palazuelos CM, Martín Parra JI, et al. New technique of transanal proctectomy with completely robotic total mesorrectal excision for rectal cancer. Cir Esp 2014;92:356-61. [PubMed]
- Simillis C, Hompes R, Penna M, et al. A systematic review of transanal total mesorectal excision: is this the future of rectal cancer surgery? Colorectal Dis 2016;18:19-36. [Crossref] [PubMed]
- Buchs NC, Nicholson GA, Ris F, et al. Transanal total mesorectal excision: A valid option for rectal cancer? World J Gastroenterol 2015;21:11700-8. [Crossref] [PubMed]
- Motson RW, Lacy A. The Rationale for Transanal Total Mesorectal Excision. Dis Colon Rectum 2015;58:911-3. [Crossref] [PubMed]
- Martínez-Pérez A, Brunetti F, de'Angelis N. Commentary on "Transanal total mesorectal excision (taTME) for rectal cancer: a systematic review and meta-analysis of oncological and perioperative outcomes compared with laparoscopic total mesorectal excision", published in BMC Cancer 2016 Jul 4;16(1):380. doi:
10.1186/s12885-016-2428-5 . Tech Coloproctol 2016;20:799-800. - Deijen CL, Velthuis S, Tsai A, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 2016;30:3210-5. [Crossref] [PubMed]
- Chen CC, Lai YL, Jiang JK, et al. Transanal Total Mesorectal Excision Versus Laparoscopic Surgery for Rectal Cancer Receiving Neoadjuvant Chemoradiation: A Matched Case-Control Study. Ann Surg Oncol 2016;23:1169-76. [Crossref] [PubMed]
- Denost Q, Adam JP, Rullier A, et al. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg 2014;260:993-9. [Crossref] [PubMed]
Cite this article as: Han J, Kim NK. Transanal approach for rectal tumors: recent updates and future perspectives. Ann Laparosc Endosc Surg 2016;1:35.


