Selection of patients with indications for robotic surgery based on personalized anatomical variations
The use of minimally invasive surgery has rapidly increased with the development of endoscopic devices and procedures. Laparoscopic gastrectomy, including laparoscopic total gastrectomy and laparoscopic proximal gastrectomy, are frequently performed especially in East Asian countries. In these conventional laparoscopic surgical procedures, several endoscopic systems provide a magnified view with full high-definition images. Therefore, surgeons can recognize anatomical microstructures such as small vessels and nerves, leading to the meticulous performance of surgical procedures. Laparoscopic surgeons, however, must manipulate long-shaft instruments fixed with ports under a two-dimensional view in these conventional laparoscopic procedures.
Robot-assisted surgery provides two main advantages: a three-dimensional (3D) view and flexibility of instrumentation. Many surgeons have considered that such advantages of robotic systems make the performance of minimally invasive surgery more meticulous and may improve short- and/or long-term surgical outcomes. According to some previous studies, however, the benefits of robotic surgery over laparoscopic surgery remain unclear (1,2). Kim et al. (2) identified no significant differences in postoperative outcomes between robotic and laparoscopic gastrectomy. Although their study confirmed the feasibility and safety of performing robotic gastrectomy, robotic surgery was associated with significantly longer operation times and greater financial costs than laparoscopic surgery. Furthermore, a 3D laparoscopic system with full high-definition images was recently developed for conventional laparoscopic surgery, and its use has rapidly increased. The advantage of the 3D view in robotic surgery will soon disappear. Therefore, we should focus on the other advantages of robotic surgery, such as the flexibility of instrumentation.
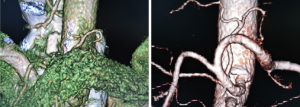
It seems that the flexibility of instrumentation in robotic surgery has dramatically improved the precision and safety of complicated procedures. However, no significant differences in short- and long-term outcomes between robotic and laparoscopic gastrectomy have been identified. In fact, some expert laparoscopic surgeons often perform high-quality laparoscopic gastrectomy, including D2 resection and/or laparoscopic total gastrectomy, that has no room for improvement using robotic surgery. Recent data may reflect this high-quality laparoscopic surgery performed by experts. However, even experts cannot always perform laparoscopic gastrectomy with the same quality as robotic surgery in all patients with gastric cancer. For example, each patient has specific anatomical variations in the celiac artery, common hepatic artery, splenic artery, and pancreas, and these variations affect the quality of suprapancreatic lymph node (LN) dissection. Figure 1 shows a 3D simulation of the suprapancreatic view based on enhanced computed tomography images. In such cases, it is difficult to perform suprapancreatic LN dissection during laparoscopic surgery because the pancreas is positioned in front of the celiac artery and splenic artery. The surgical instruments cannot reach such a suprapancreatic region without great difficulty. Figure 2 shows a 3D simulation of the suprapancreatic view of another patient who underwent D2 LN dissection. In this case, the suprapancreatic region was viewed through the laparoscope without rolling or pushing the pancreas, and the surgical instruments could easily reach this region without the flexibility provided by robotic surgery. Most experts can perform high-quality laparoscopic surgery in such patients (total gastrectomy with D2 LN dissection) without the need for improvement by robotic surgery. As shown in Figures 1 and 2, the recent development of 3D simulation provides surgeons with precise preoperative information about patients’ individual anatomical variations. Therefore, surgeons can gain a preoperative understanding of these specific anatomical variations, which affect the difficulties encountered in gastrectomy, and decide which patients are suitable for laparoscopic surgery or robotic surgery. Considering the cost-effectiveness of the use of robots, it may be better to select patients with specific anatomical variations that are not suitable for laparoscopic surgery as well as patients with certain tumor conditions. Regardless of early/advanced cancer, D1+/D2 LN dissection, or distal/total gastrectomy, a subset of patients has specific anatomical variations suitable for laparoscopic surgery while another subset of patients has variations that may be more suitable for robotic surgery.
The focus of cancer treatment worldwide is moving toward personalized therapy with anticancer reagents. In gastric surgery, it may be time to establish personalized surgeries based on preoperative information regarding individual anatomical variations and tumor conditions. In open surgery for gastric cancer, surgeons always modify their approach based on the intraoperative findings. The procedures of laparoscopic gastrectomy have recently been standardized in each institution, but some experts have several different approaches for the same procedures and select the most suitable approach among them based on the intraoperative findings. Using preoperative 3D simulation, surgeons can now intuitively obtain intraoperative images before surgery. Using such 3D simulation images, expert surgeons can preoperatively select the most suitable approach among several of their own approaches in conventional laparoscopic surgery. In addition, these experts can evaluate the difficulties in laparoscopic surgery for each patient with gastric cancer using preoperative 3D simulation images. Therefore, the difficulties in laparoscopic procedures can now be evaluated preoperatively, and the indication for robotic surgery can be determined in each case considering the cost-effectiveness of this technique. In some patients with anatomical variations suitable for laparoscopic surgery, robotic surgery does not contribute to the patients who may pay part of its cost. Thus, only patients who definitively require robotic surgery should be selected for this procedure based on their personalized anatomical variations.
Acknowledgments
Funding: This work was supported in part by Scientific Research on Innovative Areas (grant number: 26108010).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tokunaga M, Sugisawa N, Kondo J, et al. Early phase II study of robot-assisted distal gastrectomy with nodal dissection for clinical stage IA gastric cancer. Gastric Cancer 2014;17:542-7. [Crossref] [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2016;263:103-9. [Crossref] [PubMed]
Cite this article as: Ohuchida K, Nakamura M, Hashizume M. Selection of patients with indications for robotic surgery based on personalized anatomical variations. Ann Laparosc Endosc Surg 2016;1:7.